Tulipalin A
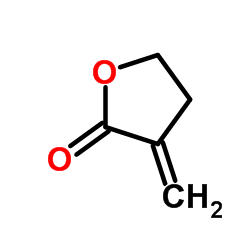
Tulipalin A structure
|
Common Name | Tulipalin A | ||
|---|---|---|---|---|
| CAS Number | 547-65-9 | Molecular Weight | 98.100 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 204.4±0.0 °C at 760 mmHg | |
| Molecular Formula | C5H6O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 37.2±0.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Warning | |
|
Design and synthesis of spiro derivatives of parthenin as novel anti-cancer agents.
Eur. J. Med. Chem. 46 , 3210-7, (2011) Several novel spiro derivatives of parthenin (1) have been synthesized by the dipolar cycloaddition using various dipoles viz; benzonitrile oxides, nitrones and azides with exocyclic double bond of C ring (α-methylene-γ-butyrolactone). Majority of the compoun... |
|
|
Construction of spiro-fused 2-oxindole/α-methylene- γ-butyrolactone systems with extremely high enantioselectivity via indium-catalyzed amide allylation of N-methyl isatin.
Org. Lett. 15(24) , 6182-5, (2013) A remarkably effective method allowing an extremely high enantioselective synthesis of the spiro-fused 2-oxindole/α-methylene-γ-butyrolactones is described. The key strategy lies in the use of indium-catalyzed asymmetric amide allylation of N-methyl isatin wi... |
|
|
Highly stereoselective synthesis of (Z)- and (E)-chloro-substituted-α-methylene-γ-butyrolactone by possibly controlling cis- and trans-chloropalladation.
Mol. Divers. 17(1) , 1-7, (2013) A palladium-catalyzed selective synthesis of cis- or trans-chloro-substituted-α-methylene-γ-butyrolactones from single substrate (propiolic acid) has been realized by controlling cis- or trans-chloropalladation. This method is highly effective for building C-... |
|
|
Structure-activity relationships of tulipalines, tuliposides, and related compounds as inhibitors of MurA.
Bioorg. Med. Chem. Lett. 20 , 5757-62, (2010) The enzyme MurA performs an essential step in peptidoglycan biosynthesis and is therefore a target for the discovery of novel antibacterial compounds. We report here the inhibition of MurA by natural products from tulips (tulipalines and tuliposides), and the... |
|
|
Free radical copolymerization kinetics of γ-methyl-α-methylene-γ-butyrolactone (MeMBL).
Biomacromolecules 12(6) , 2319-26, (2011) The propagation kinetics and copolymerization behavior of the biorenewable monomer γ-methyl-α-methylene-γ-butyrolactone (MeMBL) are studied using the pulsed laser polymerization (PLP)/size exclusion chromatography (SEC) technique. The propagation rate coeffic... |
|
|
Thermoplastic elastomers derived from menthide and tulipalin A.
Biomacromolecules 13(11) , 3833-40, (2012) Renewable ABA triblock copolymers were prepared by sequential polymerization of the plant-based monomers menthide and α-methylene-γ-butyrolactone (MBL or tulipalin A). Ring-opening transesterification polymerization of menthide using diethylene glycol as an i... |
|
|
Zinc or indium-mediated Barbier-type allylation of aldehydes with 3-bromomethyl-5H-furan-2-one in aqueous media: an efficient synthesis method for α-methylene-γ-butyrolactone.
Org. Biomol. Chem. 10(20) , 3991-8, (2012) A zinc or indium-mediated Barbier-type allylation of aldehydes with 3-bromomethyl-5H-furan-2-one in aqueous solvents was developed to provide an efficient route to α-methylene-γ-butyrolactone, which is synthetically very useful. The desired products were obta... |
|
|
CrgA is an inducible LysR-type regulator of Neisseria meningitidis, acting both as a repressor and as an activator of gene transcription.
J. Bacteriol. 187(10) , 3421-30, (2005) The crgA gene of Neisseria meningitidis, which codes for a LysR-type regulator, is divergently oriented with respect to the mdaB gene, which codes for a hypothetical NADPH-quinone oxidoreductase. Transcriptional studies of the intergenic region between crgA a... |
|
|
A novel lactone-forming carboxylesterase: molecular identification of a tuliposide A-converting enzyme in tulip.
Plant Physiol. 159(2) , 565-78, (2012) Tuliposides, the glucose esters of 4-hydroxy-2-methylenebutanoate and 3,4-dihydroxy-2-methylenebutanoate, are major secondary metabolites in tulip (Tulipa gesneriana). Their lactonized aglycons, tulipalins, function as defensive chemicals due to their biologi... |
|
|
Synthesis and cytotoxic evaluation of alpha-methylene-gamma-butyrolactone bearing naphthalene and naphtho[2,1-b]furan derivatives.
Eur. J. Med. Chem. 37(4) , 333-8, (2002) Naphthalene alpha-methylene-gamma-butyrolactones exhibits a unique cytotoxicity profile. They are highly cytostatic for leukaemia cancer cells but are not cytocidal. For almost all the solid tumours tested, they are both cytostatic and cytocidal. Substitution... |