Zinc or indium-mediated Barbier-type allylation of aldehydes with 3-bromomethyl-5H-furan-2-one in aqueous media: an efficient synthesis method for α-methylene-γ-butyrolactone.
YuZhe Gao, Xue Wang, LiDong Sun, LongGuan Xie, XiaoHua Xu
Index: Org. Biomol. Chem. 10(20) , 3991-8, (2012)
Full Text: HTML
Abstract
A zinc or indium-mediated Barbier-type allylation of aldehydes with 3-bromomethyl-5H-furan-2-one in aqueous solvents was developed to provide an efficient route to α-methylene-γ-butyrolactone, which is synthetically very useful. The desired products were obtained in moderate to high yields in aqueous solvents. Excellent drs were achieved, among which the best diastereomeric ratios of products were found when water was used in the indium-mediated reaction, and THF-NH(4)Cl (sat, aq) (2 : 1) mixture in the zinc-mediated reaction. Furthermore, the allylation can be induced by chiral centers, especially those in the α-position, as a substrate-controlled reaction to obtain the enantioselective homoallylation alcohols.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
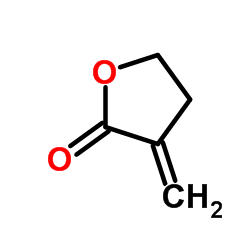 |
Tulipalin A
CAS:547-65-9 |
C5H6O2 |
|
Design and synthesis of spiro derivatives of parthenin as no...
2011-08-01 [Eur. J. Med. Chem. 46 , 3210-7, (2011)] |
|
Construction of spiro-fused 2-oxindole/α-methylene- γ-butyro...
2013-12-20 [Org. Lett. 15(24) , 6182-5, (2013)] |
|
Highly stereoselective synthesis of (Z)- and (E)-chloro-subs...
2013-02-01 [Mol. Divers. 17(1) , 1-7, (2013)] |
|
Structure-activity relationships of tulipalines, tuliposides...
2010-10-01 [Bioorg. Med. Chem. Lett. 20 , 5757-62, (2010)] |
|
Free radical copolymerization kinetics of γ-methyl-α-methyle...
2011-06-13 [Biomacromolecules 12(6) , 2319-26, (2011)] |