| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Indium
CAS:7440-74-6 |
|
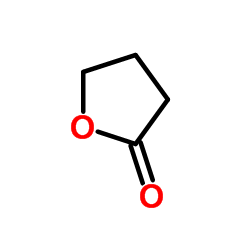 |
gamma-Butyrolactone
CAS:96-48-0 |
|
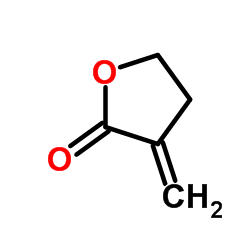 |
Tulipalin A
CAS:547-65-9 |
|
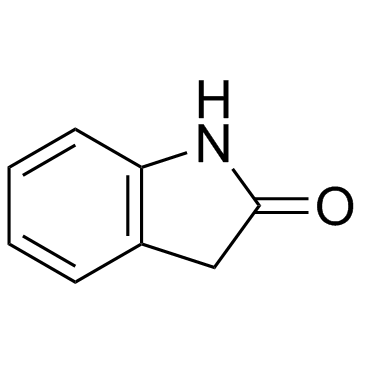 |
Oxindole
CAS:59-48-3 |
|
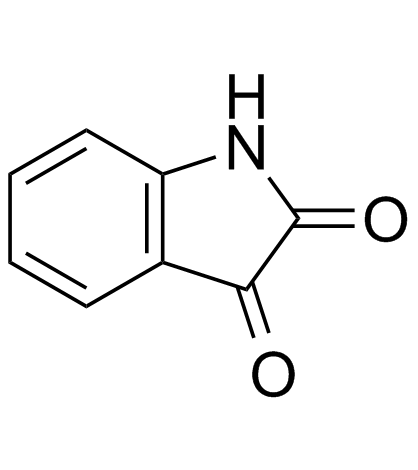 |
isatin
CAS:91-56-5 |