Oxindole
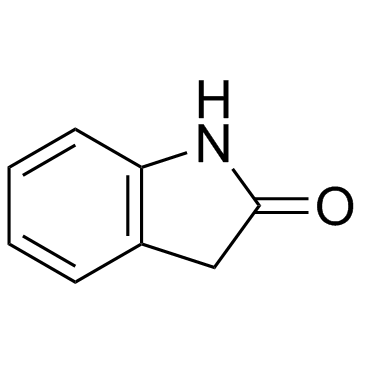
Oxindole structure
|
Common Name | Oxindole | ||
|---|---|---|---|---|
| CAS Number | 59-48-3 | Molecular Weight | 133.147 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 312.8±45.0 °C at 760 mmHg | |
| Molecular Formula | C8H7NO | Melting Point | 123-128 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 190.0±18.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of OxindoleOxindole (Indolin-2-one) is an aromatic heterocyclic building block. 2-indolinone derivatives have become lead compounds in the research of kinase inhibitors. |
| Name | indolin-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | Oxindole (Indolin-2-one) is an aromatic heterocyclic building block. 2-indolinone derivatives have become lead compounds in the research of kinase inhibitors. |
|---|---|
| Related Catalog | |
| In Vitro | Oxindole (Indolin-2-one) is a bicyclic structure consisting of a benzene ring fused to 2-pyrrolidone. Substituted indolinones can be referred as ‘privileged structures’ owing to their excellent binding affinity for many receptors and to the number of approved drugs containing this scaffold[1]. Oxindole has been found in tissues and fluids of mammals as well as natural products produced by a range of plants, bacteria and invertebrates. 2-indolinone derivatives possess diverse range of pharmacological activities including anti-cancer, anti-HIV, anti-diabetic, antibacterial, antioxidant, kinase inhibitory, AChE inhibitory, anti-leishmanial, b3 adrenergic receptor agonistic, phosphatase inhibitory, analgesic, spermicidal, vasopressin antagonists, progesterone antagonists, neuroprotection, NMDA blocker and sleep inducing activities[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 312.8±45.0 °C at 760 mmHg |
| Melting Point | 123-128 °C(lit.) |
| Molecular Formula | C8H7NO |
| Molecular Weight | 133.147 |
| Flash Point | 190.0±18.0 °C |
| Exact Mass | 133.052765 |
| PSA | 29.10000 |
| LogP | 0.61 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.640 |
| InChIKey | JYGFTBXVXVMTGB-UHFFFAOYSA-N |
| SMILES | O=C1Cc2ccccc2N1 |
| Storage condition | Refrigerator |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| RTECS | NM2080500 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 29337900 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |
|
Cytotoxic and antimicrobial evaluations of novel apoptotic and anti-angiogenic spiro cyclic 2-oxindole derivatives of 2-amino-tetrahydroquinolin-5-one.
Arch. Pharm. (Weinheim) 348(2) , 113-24, (2015) A novel series of cyclic 2-oxindole derivatives incorporating 2-amino-tetrahydroquinolin-5-one were prepared. The structures of the prepared compounds were elucidated using different spectral tools. T... |
|
|
Synthesis and biological evaluation of new pyridone-annelated isoindigos as anti-proliferative agents.
Molecules 19(9) , 13076-92, (2014) A selected set of substituted pyridone-annelated isoindigos 3a-f has been synthesized via interaction of 5- and 6-substituted oxindoles 2a-f with 6-ethyl-1,2,9-trioxopyrrolo[3,2-f]quinoline-8-carboxyl... |
|
|
Synthesis and receptor binding assay of indolin-2-one derivatives as dopamine D4 receptor ligands.
Pharmazie 70 , 511-4, (2015) Five indolin-2-one derivatives bearing piperazinylbutyl side chains attached to the amide nitrogen were synthesized from 2-indolinone. 1-(4-Bromobutyl)-indolin-2-one was reacted with 1-piperazinecarbo... |
| Oxindole |
| 3H-Indol-2-ol |
| 2-INDOLINONE |
| 2-indolinon |
| 2-Oxindoline |
| 2-oxo-indoline |
| EINECS 200-429-5 |
| OXINDOL |
| indolinone |
| 1,3-Dihydro-2H-indol-2-one |
| 2-Indolone |
| 2H-Indol-2-one, 1,3-dihydro- |
| Indolin-2-one |
| 2-Oxiindole |
| MFCD00005711 |
| 2-Oxindole |
| 1,3-dihydro-indol-2-one |
 CAS#:91-56-5
CAS#:91-56-5 CAS#:120-72-9
CAS#:120-72-9 CAS#:30095-98-8
CAS#:30095-98-8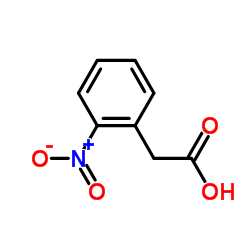 CAS#:3740-52-1
CAS#:3740-52-1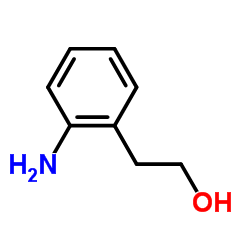 CAS#:5339-85-5
CAS#:5339-85-5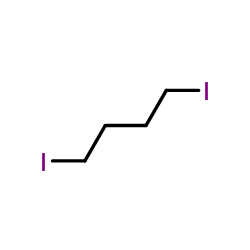 CAS#:628-21-7
CAS#:628-21-7 CAS#:109-72-8
CAS#:109-72-8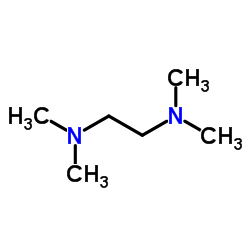 CAS#:110-18-9
CAS#:110-18-9 CAS#:2365-44-8
CAS#:2365-44-8 CAS#:65816-14-0
CAS#:65816-14-0 CAS#:496-30-0
CAS#:496-30-0 CAS#:496-15-1
CAS#:496-15-1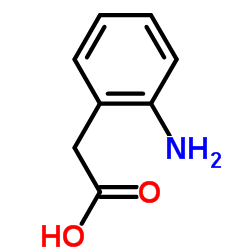 CAS#:3342-78-7
CAS#:3342-78-7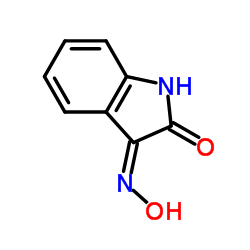 CAS#:607-28-3
CAS#:607-28-3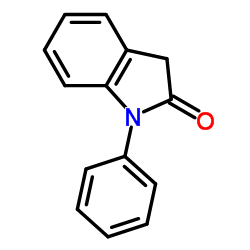 CAS#:3335-98-6
CAS#:3335-98-6 CAS#:3416-18-0
CAS#:3416-18-0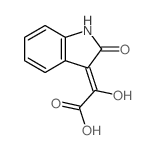 CAS#:14370-71-9
CAS#:14370-71-9 CAS#:141210-63-1
CAS#:141210-63-1 CAS#:479-41-4
CAS#:479-41-4
