European Journal of Medicinal Chemistry
2002-04-01
Synthesis and cytotoxic evaluation of alpha-methylene-gamma-butyrolactone bearing naphthalene and naphtho[2,1-b]furan derivatives.
Kuan-Han Lee, Bor-Ruey Huang
Index: Eur. J. Med. Chem. 37(4) , 333-8, (2002)
Full Text: HTML
Abstract
Naphthalene alpha-methylene-gamma-butyrolactones exhibits a unique cytotoxicity profile. They are highly cytostatic for leukaemia cancer cells but are not cytocidal. For almost all the solid tumours tested, they are both cytostatic and cytocidal. Substitution of a bromo atom on either naphthalene or both naphthalene and gamma-phenyl moiety of the lactone enhanced potency while retaining the same cytotoxicity profile. The tricyclic naphtho[2,1-b]furan derivatives, prepared from 2-hydroxy-1-naphthaldehyde in an efficient pathway, also possess the same cytotoxicity profile.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
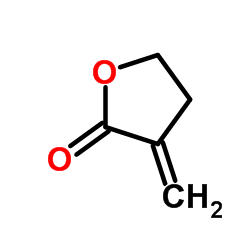 |
Tulipalin A
CAS:547-65-9 |
C5H6O2 |
Related Articles:
More...
|
Design and synthesis of spiro derivatives of parthenin as no...
2011-08-01 [Eur. J. Med. Chem. 46 , 3210-7, (2011)] |
|
Construction of spiro-fused 2-oxindole/α-methylene- γ-butyro...
2013-12-20 [Org. Lett. 15(24) , 6182-5, (2013)] |
|
Highly stereoselective synthesis of (Z)- and (E)-chloro-subs...
2013-02-01 [Mol. Divers. 17(1) , 1-7, (2013)] |
|
Structure-activity relationships of tulipalines, tuliposides...
2010-10-01 [Bioorg. Med. Chem. Lett. 20 , 5757-62, (2010)] |
|
Free radical copolymerization kinetics of γ-methyl-α-methyle...
2011-06-13 [Biomacromolecules 12(6) , 2319-26, (2011)] |