A novel lactone-forming carboxylesterase: molecular identification of a tuliposide A-converting enzyme in tulip.
Taiji Nomura, Shinjiro Ogita, Yasuo Kato
Index: Plant Physiol. 159(2) , 565-78, (2012)
Full Text: HTML
Abstract
Tuliposides, the glucose esters of 4-hydroxy-2-methylenebutanoate and 3,4-dihydroxy-2-methylenebutanoate, are major secondary metabolites in tulip (Tulipa gesneriana). Their lactonized aglycons, tulipalins, function as defensive chemicals due to their biological activities. We recently found that tuliposide-converting enzyme (TCE) purified from tulip bulbs catalyzed the conversion of tuliposides to tulipalins, but the possibility of the presence of several TCE isozymes was raised: TCE in tissues other than bulbs is different from bulb TCE. Here, to prove this hypothesis, TCE was purified from petals, which have the second highest TCE activity after bulbs. The purified enzyme, like the bulb enzyme, preferentially accepted tuliposides as substrates, with 6-tuliposide A the best substrate, which allowed naming the enzyme tuliposide A-converting enzyme (TCEA), but specific activity and molecular mass differed between the petal and bulb enzymes. After peptide sequencing, a novel cDNA (TgTCEA) encoding petal TCEA was isolated, and the functional characterization of the recombinant enzyme verified that TgTCEA catalyzes the conversion of 6-tuliposide A to tulipalin A. TgTCEA was transcribed in all tulip tissues but not in bulbs, indicating the presence of a bulb-specific TgTCEA, as suggested by the distinct enzymatic characters between the petal and bulb enzymes. Plastidial localization of TgTCEA enzyme was revealed, which allowed proposing a cytological mechanism of TgTCE-mediated tulipalin formation in the tulip defensive strategy. Site-directed mutagenesis of TgTCEA suggested that the oxyanion hole and catalytic triad characteristic of typical carboxylesterases are essential for the catalytic process of TgTCEA enzyme. To our knowledge, TgTCEA is the first identified member of the lactone-forming carboxylesterases, specifically catalyzing intramolecular transesterification.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
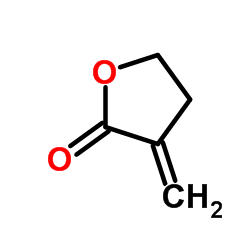 |
Tulipalin A
CAS:547-65-9 |
C5H6O2 |
|
Design and synthesis of spiro derivatives of parthenin as no...
2011-08-01 [Eur. J. Med. Chem. 46 , 3210-7, (2011)] |
|
Construction of spiro-fused 2-oxindole/α-methylene- γ-butyro...
2013-12-20 [Org. Lett. 15(24) , 6182-5, (2013)] |
|
Highly stereoselective synthesis of (Z)- and (E)-chloro-subs...
2013-02-01 [Mol. Divers. 17(1) , 1-7, (2013)] |
|
Structure-activity relationships of tulipalines, tuliposides...
2010-10-01 [Bioorg. Med. Chem. Lett. 20 , 5757-62, (2010)] |
|
Free radical copolymerization kinetics of γ-methyl-α-methyle...
2011-06-13 [Biomacromolecules 12(6) , 2319-26, (2011)] |