6-Chloroguanineriboside
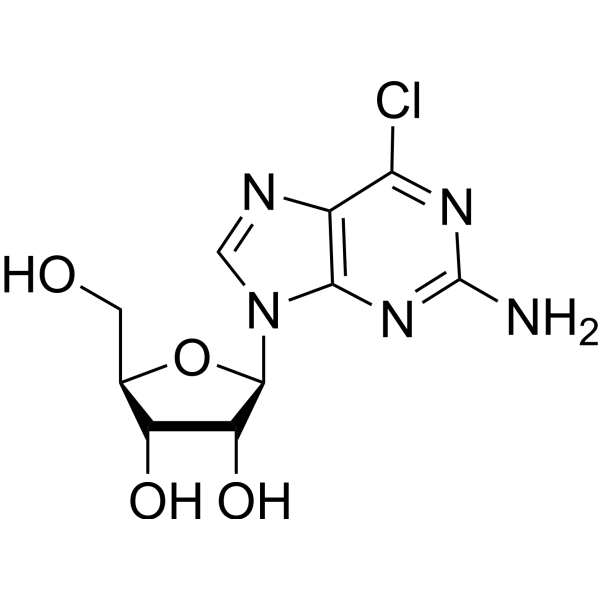
6-Chloroguanineriboside structure
|
Common Name | 6-Chloroguanineriboside | ||
|---|---|---|---|---|
| CAS Number | 2004-07-1 | Molecular Weight | 301.69 | |
| Density | 2.2±0.1 g/cm3 | Boiling Point | 729.9±70.0 °C at 760 mmHg | |
| Molecular Formula | C10H12ClN5O4 | Melting Point | 165-167 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 395.2±35.7 °C | |
Use of 6-Chloroguanineriboside6-Chloroguanineriboside (6-Chloroguanosine) is a purine nucleoside analogue. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
| Name | 2-Amino-6-chloropurine-9-riboside |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Chloroguanineriboside (6-Chloroguanosine) is a purine nucleoside analogue. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 2.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 729.9±70.0 °C at 760 mmHg |
| Melting Point | 165-167 °C (dec.)(lit.) |
| Molecular Formula | C10H12ClN5O4 |
| Molecular Weight | 301.69 |
| Flash Point | 395.2±35.7 °C |
| Exact Mass | 301.057770 |
| PSA | 139.54000 |
| LogP | -0.59 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.912 |
| InChIKey | TXWHPSZYRUHEGT-VKJDSPIKSA-N |
| SMILES | Nc1nc(Cl)c2ncn(C3OC(CO)C(O)C3O)c2n1 |
| Storage condition | −20°C |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
|
Preparative electrochemical reduction of 2-amino-6-chloropurine and synthesis of 6-deoxyacyclovir, a fluorescent substrate of xanthine oxidase and a prodrug of acyclovir.
Acta Chem. Scand. B 41 , 701-707, (1987) D.c. polarography of 2-amino-6-chloropurine in aqueous medium over a broad pH range revealed two diffusion waves, the first of which corresponds to reduction of the C(6)-Cl bond, leading to formation ... |
|
|
Synthesis of a mutagenic nucleoside, 2'-deoxy-2-(p-nitrophenyl)-adenosine.
Nucleic Acids Symp. Ser. 17 , 141-143, (1986) The reaction of 2-amino-6-chloropurine riboside with i-amyl nitrite in benzene in the presence of Cu2O, followed by treatment with NH3/MeOH gave 2-phenyladenosine (1). The crude sample of 1 was found ... |
|
|
Synthesis of 6-arylthio analogs of 2',3'-dideoxy-3'-fluoroguanosine and their effect against hepatitis B virus replication.
Nucleosides Nucleotides Nucleic Acids 25 , 655-665, (2006) A key compound, 2-amino-6-chlor-9-(2,3-dideoxy-3-fluoro-beta-D-erythro-pentofuranosyl)puine, was prepared from 2-amino-6-chloropurine riboside in 5 steps, then subjected to the nucleophilic displaceme... |
| (−)-2-Amino-6-chloropurine riboside 6-Chloroguanine riboside |
| 6-Chloroguanineriboside |
| 2-Amino-6-chloro-9-(β-D-ribofuranosyl)purine |
| 6-Chloro-9-(β-D-ribofuranosyl)-9H-purin-2-amine |
| EINECS 217-905-3 |
| (−)-2-Amino-6-chloropurine riboside |
| 6-CHLOROGUANOSINE |
| 2-Amino-6-chloropurine Riboside |
| 6-Chloroguanine nucleoside |
| 6-CHLOROADENOSINE |
| 6-Chloroguanosine2 |
| MFCD00005735 |
| 2-Amino-6-chloropurine-9--D-riboside |
| 9H-Purin-2-amine, 6-chloro-9-β-D-ribofuranosyl- |
| 6-Chloroguanine riboside |
| 2-amino-6-chloro purineriboside |
 CAS#:16321-99-6
CAS#:16321-99-6 CAS#:108321-99-9
CAS#:108321-99-9 CAS#:10310-21-1
CAS#:10310-21-1 CAS#:81100-62-1
CAS#:81100-62-1 CAS#:118-00-3
CAS#:118-00-3 CAS#:6979-94-8
CAS#:6979-94-8 CAS#:67-56-1
CAS#:67-56-1 CAS#:3387-63-1
CAS#:3387-63-1 CAS#:28708-32-9
CAS#:28708-32-9 CAS#:109881-25-6
CAS#:109881-25-6 CAS#:107550-73-2
CAS#:107550-73-2 CAS#:4546-54-7
CAS#:4546-54-7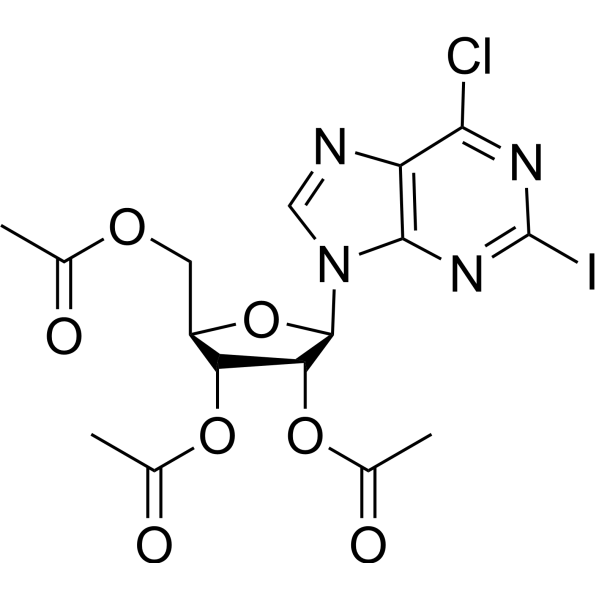 CAS#:5987-76-8
CAS#:5987-76-8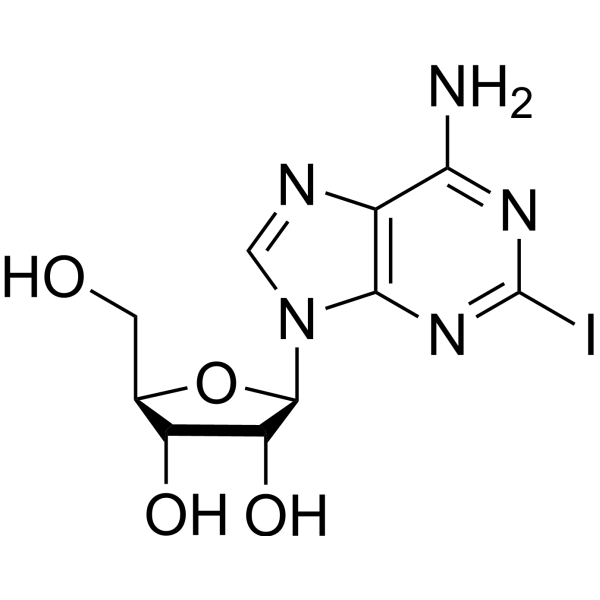 CAS#:35109-88-7
CAS#:35109-88-7 CAS#:29411-74-3
CAS#:29411-74-3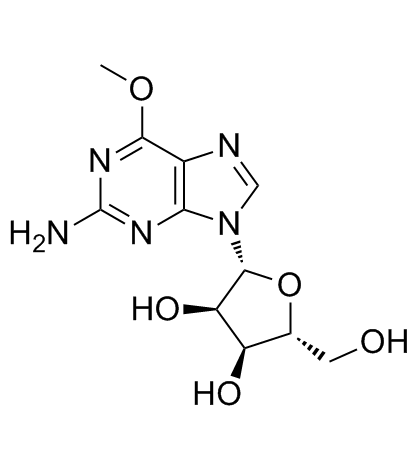 CAS#:7803-88-5
CAS#:7803-88-5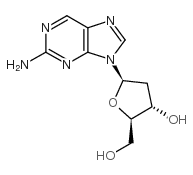 CAS#:3616-24-8
CAS#:3616-24-8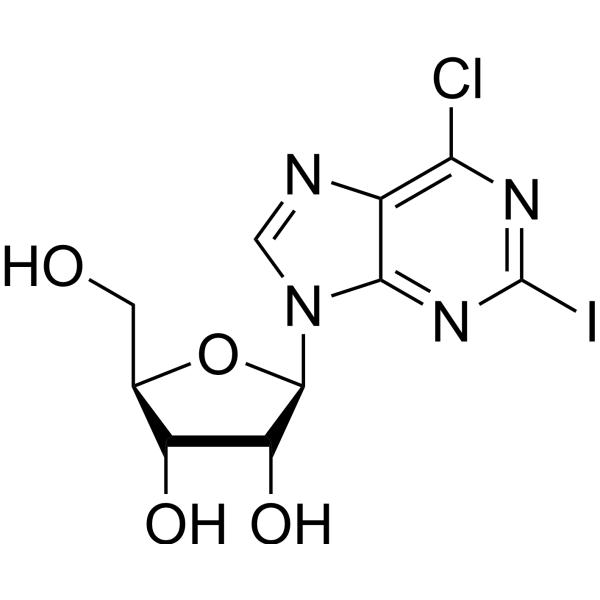 CAS#:313477-85-9
CAS#:313477-85-9
