| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
sucrose
CAS:57-50-1 |
|
 |
Isopropanol
CAS:67-63-0 |
|
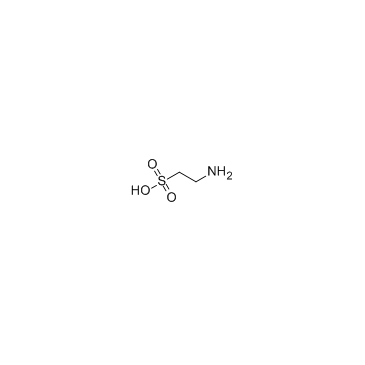 |
Taurine
CAS:107-35-7 |
|
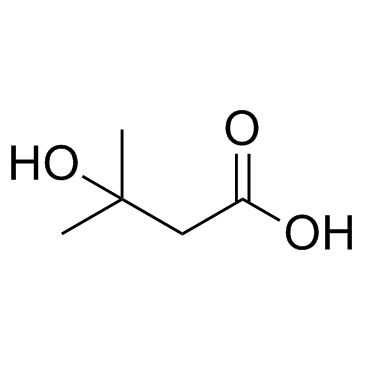 |
3-Hydroxyisovaleric acid
CAS:625-08-1 |
|
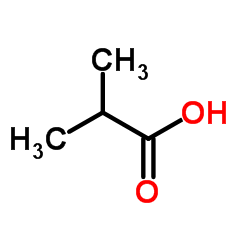 |
Isobutyric acid
CAS:79-31-2 |
|
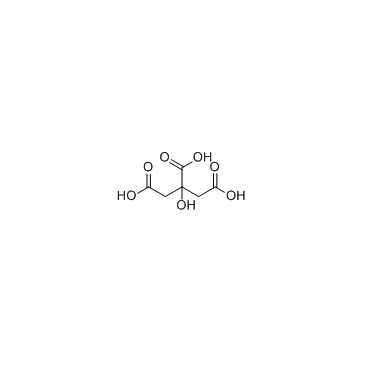 |
Citric Acid
CAS:77-92-9 |
|
 |
fumaric acid
CAS:110-17-8 |
|
 |
Succinic acid
CAS:110-15-6 |
|
 |
acetic acid
CAS:64-19-7 |