ABD-F
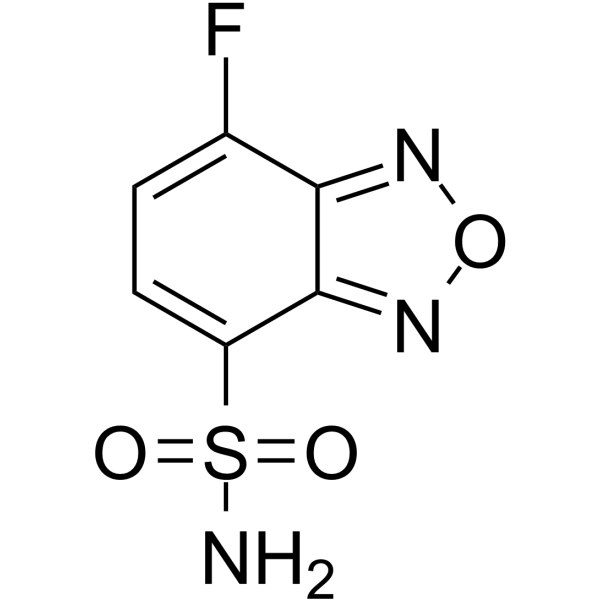
ABD-F structure
|
Common Name | ABD-F | ||
|---|---|---|---|---|
| CAS Number | 91366-65-3 | Molecular Weight | 217.178 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 408.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C6H4FN3O3S | Melting Point | 145-146ºC | |
| MSDS | USA | Flash Point | 201.0±31.5 °C | |
|
Quantification of glutathione in plasma samples by HPLC using 4-fluoro-7-nitrobenzofurazan as a fluorescent labeling reagent.
J. Chromatogr. Sci. 50 , 119-122, (2012) A rapid and highly sensitive high-performance liquid chromatograpy method with fluorescence detection has been developed for determination of glutathione (GSH) in human plasma. A simple pre-column derivatization procedure with 7-flouro-4-nitrobenzo-2-oxa-1,3-... |
|
|
Plasma thiols levels in Alzheimer's disease mice under diet-induced hyperhomocysteinemia: effect of S-adenosylmethionine and superoxide-dismutase supplementation.
J. Alzheimers Dis. 44(4) , 1323-31, (2015) Widely confirmed reports were published on association between hyperhomocysteinemia, B vitamin deficiency, oxidative stress, and amyloid-β in Alzheimer's disease (AD). Homocysteine, cysteine, cysteinylglycine and glutathione are metabolically interrelated thi... |
|
|
Allosteric inhibition of PTP1B activity by selective modification of a non-active site cysteine residue.
Biochemistry 44(21) , 7704-12, (2005) The fluorogenic reagent 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole (ABDF) attenuates the functional activity of the protein tyrosine phosphatase PTP1B by reacting selectively with a single cysteine residue, leaving other cysteines in the protein unmodifi... |
|
|
Characterization of cysteine residues of glutathione S-transferase P: evidence for steric hindrance of substrate binding by a bulky adduct to cysteine 47.
Biochem. Biophys. Res. Commun. 188(1) , 424-32, (1992) Glutathione S-transferase P (GST-P) lost the enzymatic activity by 7-fluoro-4-sulfamoyl-2, 1, 3-benzodiazole (ABD-F), a thiol-group chemical modifier, but did not by methylmethanethiol-sulfonate. Both ABD-F and methylmethanethiolsulfonate reacted with Cys47 a... |
|
|
Antichymotrypsin interaction with chymotrypsin. Intermediates on the way to inhibited complex formation.
J. Biol. Chem. 274(25) , 17733-41, (1999) Serpins form enzymatically inactive covalent complexes (designated E*I*) with their target proteinases, corresponding most likely to the acyl enzyme that resembles the normal intermediate in substrate turnover. Formation of E*I* involves large changes in the ... |
|
|
Removal of the fluorescent 4-(aminosulfonyl)-2,1,3-benzoxadiazole label from cysteine-containing peptides.
J. Chromatogr. A. 798(1-2) , 47-54, (1998) The complete removal of the fluorescent cysteine derivative 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole (ABD-F) from an intact protein has not been demonstrated even after extended treatment with a reducing agent. It has been suggested that this may be du... |
|
|
A new HPLC micromethod to measure total plasma homocysteine in newborn.
J. Pharm. Biomed. Anal. 24(5-6) , 1137-41, (2001) Total plasma homocysteine (tHcy) in children may be an useful biochemical marker for genetic risk of premature cardiovascular disease. We reported a rapid, isocratic HPLC method able to process very small amount of newborn plasma samples. A blood sample from ... |
|
|
Structural analysis of the sheath of a sheathed bacterium, Leptothrix cholodnii.
Int. J. Biol. Macromol. 37(1-2) , 92-8, (2005) Leptothrix cholodnii is an aerobic sheath-forming bacterium often found in oligotrophic and metal-rich aquatic environments. The sheath of this bacterium was isolated by selectively lysing the cells. Glycine and cysteine were the major amino acids of the shea... |
|
|
Identification of 2-(cysteinyl)amido-2-deoxy-D-galacturonic acid residue from the sheath of Leptothrix cholodnii.
Biosci. Biotechnol. Biochem. 70(5) , 1265-8, (2006) The sheath of Leptothrix cholodnii is a glycoconjugate composed of a polysaccharide and a peptide rich in cysteine. In this study, structural determination of the hydrazinolyzate of the sheath was carried out. Since the hydrazinolyzate is a polysaccharide inc... |
|
|
Reversibility of cysteine labeling by 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole.
Anal. Biochem. 212(1) , 138-42, (1993) The fluorescent cysteine derivative formed upon alkylation of proteins with 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole (ABD-F) is shown to be unstable under certain conditions. Although previously thought to be an irreversible reaction, the fluorescence ... |