Characterization of cysteine residues of glutathione S-transferase P: evidence for steric hindrance of substrate binding by a bulky adduct to cysteine 47.
J Nishihira, T Ishibashi, M Sakai, S Nishi, T Kumazaki, Y Hatanaka, S Tsuda, K Hikichi
Index: Biochem. Biophys. Res. Commun. 188(1) , 424-32, (1992)
Full Text: HTML
Abstract
Glutathione S-transferase P (GST-P) lost the enzymatic activity by 7-fluoro-4-sulfamoyl-2, 1, 3-benzodiazole (ABD-F), a thiol-group chemical modifier, but did not by methylmethanethiol-sulfonate. Both ABD-F and methylmethanethiolsulfonate reacted with Cys47 and Cys101. These two cysteine residues were site-directedly mutated with serine residues. Only the Cys101Ser lost the enzymatic activity by the treatment of ABD-F. On carbon 13 NMR experiments, a NMR signal of S-[13C]CH3 adduct to Cys47 did not show any change by the addition of S-hexylglutathione. These facts revealed that Cys47 did not locate at the active site, and a bulky adduct to Cys47 hindered the binding of substrates to the active site.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
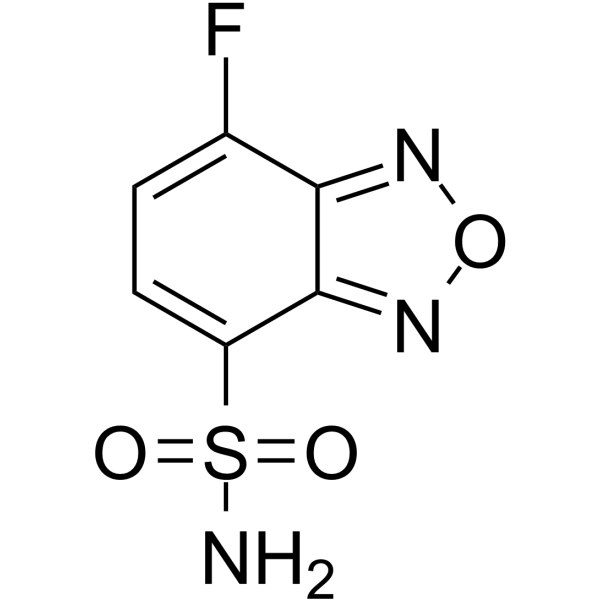 |
ABD-F
CAS:91366-65-3 |
C6H4FN3O3S |
|
Quantification of glutathione in plasma samples by HPLC usin...
2012-02-01 [J. Chromatogr. Sci. 50 , 119-122, (2012)] |
|
Plasma thiols levels in Alzheimer's disease mice under diet-...
2015-01-01 [J. Alzheimers Dis. 44(4) , 1323-31, (2015)] |
|
Allosteric inhibition of PTP1B activity by selective modific...
2005-05-31 [Biochemistry 44(21) , 7704-12, (2005)] |
|
Antichymotrypsin interaction with chymotrypsin. Intermediate...
1999-06-18 [J. Biol. Chem. 274(25) , 17733-41, (1999)] |
|
Removal of the fluorescent 4-(aminosulfonyl)-2,1,3-benzoxadi...
1998-03-06 [J. Chromatogr. A. 798(1-2) , 47-54, (1998)] |