Allosteric inhibition of PTP1B activity by selective modification of a non-active site cysteine residue.
Stig K Hansen, Mark T Cancilla, Timothy P Shiau, Jenny Kung, Teresa Chen, Daniel A Erlanson
Index: Biochemistry 44(21) , 7704-12, (2005)
Full Text: HTML
Abstract
The fluorogenic reagent 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole (ABDF) attenuates the functional activity of the protein tyrosine phosphatase PTP1B by reacting selectively with a single cysteine residue, leaving other cysteines in the protein unmodified. This modification reduces Vmax without substantially affecting substrate binding (Km), indicative of an allosteric mode of inhibition. Consistent with this, the cysteine residue modified by ABDF, Cys 121, lies outside the catalytic site but makes interactions with residues that contact His 214, which has been shown to be important for catalysis. Cys 121 is highly conserved among phosphatases, and ABDF also inhibits TC-PTP and LAR. These findings illustrate that targeting cysteine residues outside catalytic sites may be exploited in allosterically regulating enzymes. Moreover, these results suggest a new strategy for inhibiting a promising diabetes target.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
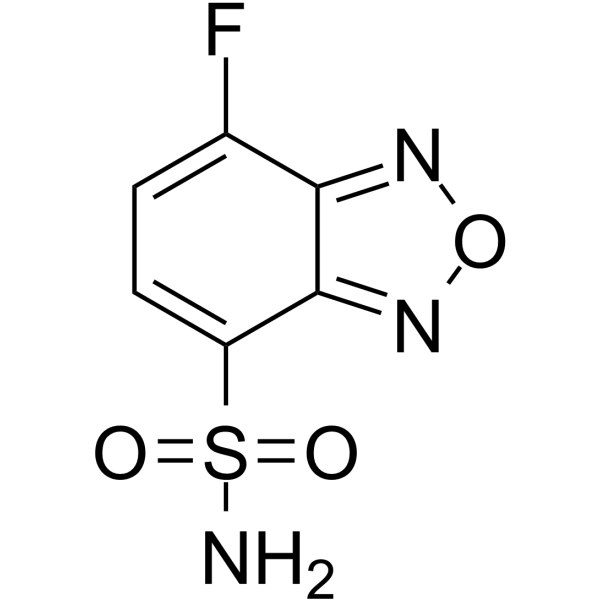 |
ABD-F
CAS:91366-65-3 |
C6H4FN3O3S |
|
Quantification of glutathione in plasma samples by HPLC usin...
2012-02-01 [J. Chromatogr. Sci. 50 , 119-122, (2012)] |
|
Plasma thiols levels in Alzheimer's disease mice under diet-...
2015-01-01 [J. Alzheimers Dis. 44(4) , 1323-31, (2015)] |
|
Characterization of cysteine residues of glutathione S-trans...
1992-10-15 [Biochem. Biophys. Res. Commun. 188(1) , 424-32, (1992)] |
|
Antichymotrypsin interaction with chymotrypsin. Intermediate...
1999-06-18 [J. Biol. Chem. 274(25) , 17733-41, (1999)] |
|
Removal of the fluorescent 4-(aminosulfonyl)-2,1,3-benzoxadi...
1998-03-06 [J. Chromatogr. A. 798(1-2) , 47-54, (1998)] |