Reversibility of cysteine labeling by 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole.
M J Treuheit, T L Kirley
Index: Anal. Biochem. 212(1) , 138-42, (1993)
Full Text: HTML
Abstract
The fluorescent cysteine derivative formed upon alkylation of proteins with 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole (ABD-F) is shown to be unstable under certain conditions. Although previously thought to be an irreversible reaction, the fluorescence and absorbance associated with the ABD-cysteine can be released from the protein by heating at basic pH in the presence of reductants. Reversal of labeling is accelerated by increasing the temperature, pH, and concentration of reductant. Upon reversal of labeling, a free sulfhydryl is regenerated, since the treated protein can subsequently be realkylated (without reductant) to approximately the same level with only ABD-F. The findings reported in this work are relevant to the use of this alkylating reagent prior to any procedure involving heating or long incubation under reducing conditions, including re-electrophoresis or enzymatic deglycosylation. Investigators using ABD-F or structurally similar reagents should be aware that the cysteine derivative formed is not stable under these conditions. The reversal of cysteine derivatization may also be useful as a method for replacing the ABD-cysteine adduct with a label more suitable for subsequent specialized procedures.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
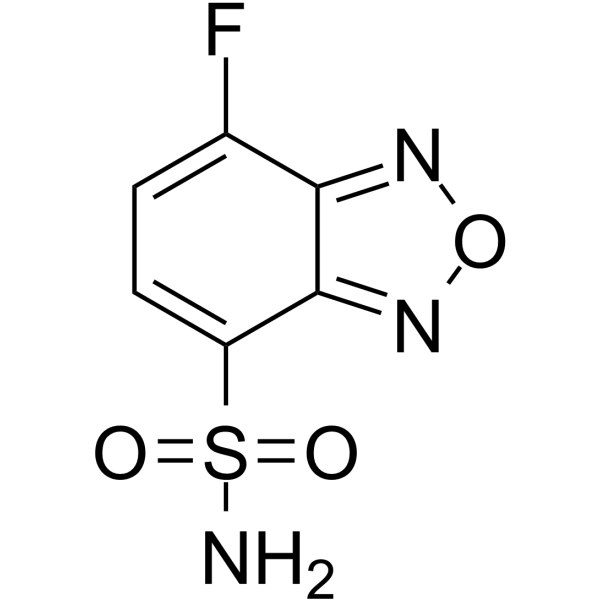 |
ABD-F
CAS:91366-65-3 |
C6H4FN3O3S |
|
Quantification of glutathione in plasma samples by HPLC usin...
2012-02-01 [J. Chromatogr. Sci. 50 , 119-122, (2012)] |
|
Plasma thiols levels in Alzheimer's disease mice under diet-...
2015-01-01 [J. Alzheimers Dis. 44(4) , 1323-31, (2015)] |
|
Allosteric inhibition of PTP1B activity by selective modific...
2005-05-31 [Biochemistry 44(21) , 7704-12, (2005)] |
|
Characterization of cysteine residues of glutathione S-trans...
1992-10-15 [Biochem. Biophys. Res. Commun. 188(1) , 424-32, (1992)] |
|
Antichymotrypsin interaction with chymotrypsin. Intermediate...
1999-06-18 [J. Biol. Chem. 274(25) , 17733-41, (1999)] |