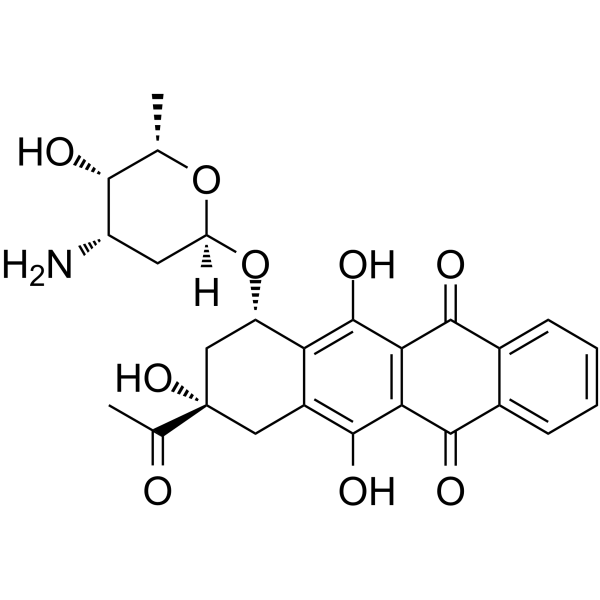
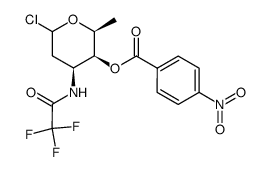
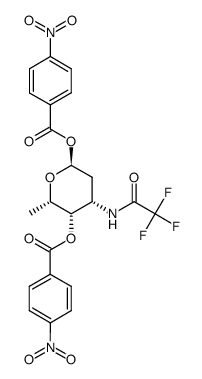
58957-92-9
| 中文名 | 伊达比星 |
|---|---|
| 英文名 | idarubicin |
| 中文别名 |
4-去甲氧基柔红霉素
(1S,3S)-3-乙酰-1,2,3,4,6,11-六氢-3,15,12-三羟基-6,11-二氧代-L-丁炔基-3-氨基-2,3,6-三脱氧基-Α-L-来苏糖吡喃己糖苷 伊达吡星 依达比星 |
| 英文别名 |
(7S-cis)-9-Acetyl-7-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-5,12-naphthacenedione
(1S,3S)-3-Acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside Idarubicine Idarubicina EINECS 260-990-7 4-Demethoxydaunomycin Idarubicine [INN-French] 5,12-naphthacenedione Idamycin Idarubicinum 4-Demethoxydaunorubicin Idarubicinum [INN-Latin] Idarubicin MFCD00866457 (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione (1S,3S)-3-Acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydro-1-tetracenyl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside |
| 描述 | 阿柔比星是一种口服有效的蒽环类抗白血病药物。阿柔比星抑制拓扑异构酶II干扰DNA和RNA转录的复制。阿柔比星可诱导DNA损伤。阿柔比星抑制DNA合成和c-myc表达。爱达霉素抑制细菌和酵母菌的生长[1][2][3][4][5]。 |
|---|---|
| 相关类别 | |
| 靶点 |
Topoisomerase II |
| 体外研究 | 在MCF-7单分子膜上,伊达柔比星的IC50为3.3±0.4 ng/mL,在多细胞球体上为7.9±1.1 ng/mL[1]。在各种体外系统中,伊达柔比星显示出比柔红霉素或阿霉素更大的细胞毒性。这归因于阿柔比星诱导拓扑异构酶II介导的DNA断裂形成的更好能力[2]。伊达阿霉素的活性分别是阿霉素和表阿霉素的57.5倍和25倍[3]。阿柔比星在MCF-7细胞生长中产生浓度依赖性降低,IC50约为0.01μM。阿柔比星对DNA合成产生浓度依赖性抑制,对c-myc表达产生时间和浓度依赖性的抑制[4]。 |
| 参考文献 |
[2]. Robert J. Clinical pharmacokinetics of idarubicin. Clin Pharmacokinet. 1993 Apr;24(4):275-88. |
| 密度 | 1.6±0.1 g/cm3 |
|---|---|
| 沸点 | 725.4±60.0 °C at 760 mmHg |
| 分子式 | C26H27NO9 |
| 分子量 | 497.494 |
| 闪点 | 392.5±32.9 °C |
| 精确质量 | 497.168579 |
| PSA | 176.61000 |
| LogP | 2.95 |
| 外观性状 | solid |
| 蒸汽压 | 0.0±2.5 mmHg at 25°C |
| 折射率 | 1.706 |
| 储存条件 | 2-8°C |
| 稳定性 | 盐酸伊达比星(Idarubicin Hydrochloride):C26H27NO9?HCl。[57852-57-0]。鲜橙色结晶性粉末,熔点183~185℃;也有报道熔点172~174℃。[α]D20+205°(C=0.1,甲醇);也有[α]D20+188°(C=0.10,甲醇)。 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:5 3.氢键受体数量:10 4.可旋转化学键数量:3 5.互变异构体数量:54 6.拓扑分子极性表面积177 7.重原子数量:36 8.表面电荷:0 9.复杂度:912 10.同位素原子数量:0 11.确定原子立构中心数量:6 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 危害码 (欧洲) | T+ |
|---|---|
| 风险声明 (欧洲) | 60-61-28-40 |
| 安全声明 (欧洲) | S53-S45 |
| 危险品运输编码 | UN 2811 6.1/PG 2 |
| WGK德国 | 3 |
| RTECS号 | HB7877000 |
| 危险类别 | 6.1 |
| 上游产品 6 | |
|---|---|
| 下游产品 0 | |