TCO-OH
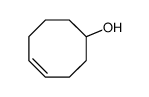
TCO-OH structure
|
Common Name | TCO-OH | ||
|---|---|---|---|---|
| CAS Number | 85081-69-2 | Molecular Weight | 126.19600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C8H14O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS05 |
Signal Word | Danger | |
Use of TCO-OHTCO-OH is an alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs[1]. |
| Name | 4-Cycloocten-1-ol, (4E) |
|---|---|
| Synonym | More Synonyms |
| Description | TCO-OH is an alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs[1]. |
|---|---|
| Related Catalog | |
| Target |
Alkyl-Chain |
| In Vitro | PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[1]. |
| References |
| Molecular Formula | C8H14O |
|---|---|
| Molecular Weight | 126.19600 |
| Exact Mass | 126.10400 |
| PSA | 20.23000 |
| LogP | 1.86760 |
| InChIKey | UCPDHOTYYDHPEN-CMLYIYFCSA-N |
| SMILES | OC1CCC=CCCC1 |
| Storage condition | 20°C |
|
Synthesis and evaluation of a series of 1,2,4,5-tetrazines for bioorthogonal conjugation.
Bioconjug. Chem. 22 , 2263-2270, (2011) 1,2,4,5-Tetrazines have been established as effective dienes for inverse electron demand [4 + 2] Diels-Alder cycloaddition reactions with strained alkenes for over 50 years. Recently, this reaction pa... |
|
|
Tetrazine-based cycloadditions: application to pretargeted live cell imaging.
Bioconjug. Chem. 19 , 2297-2299, (2008) Bioorthogonal tetrazine cycloadditions have been applied to live cell labeling. Tetrazines react irreversibly with the strained dienophile norbornene forming dihydropyrazine products and dinitrogen. T... |
|
|
Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity.
J. Am. Chem. Soc. 130 , 13518-13519, (2008) Described is a bioorthogonal reaction that proceeds with unusually fast reaction rates without need for catalysis: the cycloaddition of s-tetrazine and trans-cyclooctene derivatives. The reactions tol... |
| (E)-CYCLOOCT-4-ENOL |
| 4-Cycloocten-1-ol, (E)- |
| (E)-Cyclooct-4-enol |