| Structure | Name/CAS No. | Articles |
|---|---|---|
![5-[4-(1,2,4,5-Tetrazin-3-yl)benzylamino]-5-oxopentanoic acid Structure](https://image.chemsrc.com/caspic/144/1225146-53-1.png) |
5-[4-(1,2,4,5-Tetrazin-3-yl)benzylamino]-5-oxopentanoic acid
CAS:1225146-53-1 |
|
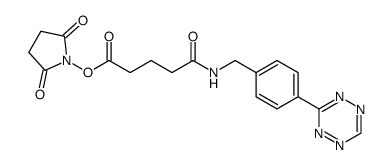 |
Tetrazine-Ph-NHCO-C3-NHS ester
CAS:1244040-64-9 |
|
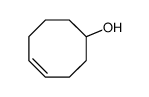 |
TCO-OH
CAS:85081-69-2 |
|
 |
TCO-NHS ester
CAS:1191901-33-3 |
|
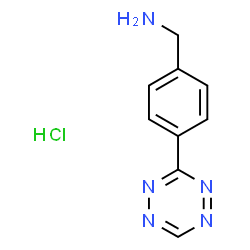 |
H-Tz-Bz-NH3Cl hydrochloride
CAS:1345866-68-3 |
|
 |
Cyclooctane
CAS:292-64-8 |