Clozapine
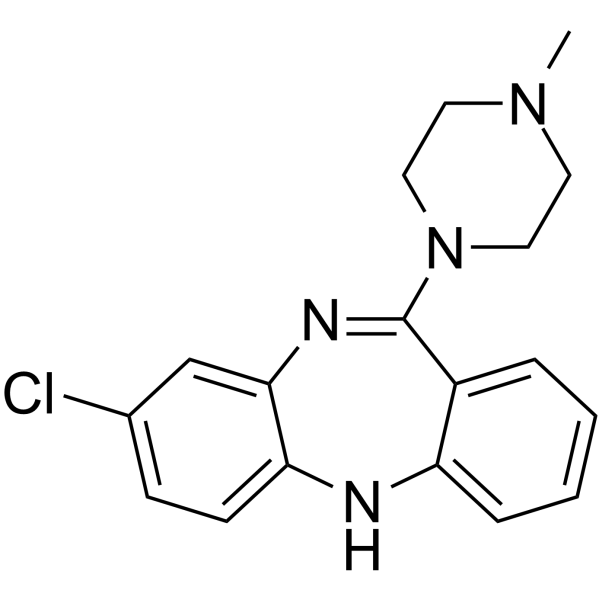
Clozapine structure
|
Common Name | Clozapine | ||
|---|---|---|---|---|
| CAS Number | 5786-21-0 | Molecular Weight | 326.823 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 489.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C18H19ClN4 | Melting Point | 182-185°C | |
| MSDS | Chinese USA | Flash Point | 249.6±31.5 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of ClozapineClozapine (HF 1854) is an antipsychotic used to treat schizophrenia. Clozapine is a potent antagonist of dopamine and a number of other receptors, with a Ki of 9.5 nM for M1 receptor. |
| Name | clozapine |
|---|---|
| Synonym | More Synonyms |
| Description | Clozapine (HF 1854) is an antipsychotic used to treat schizophrenia. Clozapine is a potent antagonist of dopamine and a number of other receptors, with a Ki of 9.5 nM for M1 receptor. |
|---|---|
| Related Catalog | |
| Target |
Ki: 9.5 nM (M1), 51 nM (α2-adrenoceptor), 75 nM (D2)[1] |
| In Vitro | Clozapine shows the unique property of having antipsychotic action but no Parkinson-like motor side effects. The chemical structure of clozapine facilitates a relatively rapid dissociation from D2 receptors. After short-term occupation of D2 receptors, peak neural activity raises synaptic dopamine, which then displaces clozapine. While clozapine also occupies other types of receptors, they may not have a significant role in preventing parkinsonism. Clozapine is very potent at D2 receptor with a Ki of 75 nM. Clozapine is also potent at the α2-adrenoceptor with a Ki value of 51 nM[1]. Clozapine causes paradoxical downregulation of 5-HT2A receptors. Clozapine also binds to 5-HT6 and 5-HT7 receptors with high affnity[2]. |
| In Vivo | Head-twitch response is decreased and [3H]ketanserin binding is downregulated in 1, 7, and 14 days after chronic clozapine. 5-HT2A mRNA is reduced 1 day after chronic clozapine. Induction of c-fos, but not egr-1 and egr-2, is rescued 7 days after chronicclozapine[3]. |
| Animal Admin | Mice: Mice are treated chronically (21 days) with 25 mg/kg/day clozapine. Experiments are conducted 1, 7, 14, and 21 days after the last clozapine administration. [3H]Ketanserin binding and 5-HT2A mRNA expression are determined in mouse somatosensory cortex. Head-twitch behavior, expression of c-fos, which is induced by all 5-HT2A agonists, and expression of egr-1 and egr-2, which are LSD-like specific, are assayed[3]. |
| References |
[1]. Seeman P, et al. Clozapine, a fast-off-D2 antipsychotic. ACS Chem Neurosci. 2014 Jan 15;5(1):24-9. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 489.2±55.0 °C at 760 mmHg |
| Melting Point | 182-185°C |
| Molecular Formula | C18H19ClN4 |
| Molecular Weight | 326.823 |
| Flash Point | 249.6±31.5 °C |
| Exact Mass | 326.129822 |
| PSA | 30.87000 |
| LogP | 2.36 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.681 |
| Storage condition | Store at RT |
| Water Solubility | ethanol: 1 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H341-H361 |
| Precautionary Statements | Missing Phrase - N15.00950417-P280 |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | HP1750000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
|
~88% 
Clozapine CAS#:5786-21-0 |
| Literature: Australian Journal of Chemistry, , vol. 60, # 9 p. 673 - 684 |
|
~41% 
Clozapine CAS#:5786-21-0 |
| Literature: US2007/92586 A1, ; Page/Page column 13 ; |
|
~21% 
Clozapine CAS#:5786-21-0 |
| Literature: US2010/166887 A1, ; US 20100166887 A1 |
|
~% 
Clozapine CAS#:5786-21-0 |
| Literature: Tetrahedron, , vol. 66, # 41 p. 8203 - 8209 |
|
~% 
Clozapine CAS#:5786-21-0 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 16, # 17 p. 4543 - 4547 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Characterization of a highly sensitive and selective novel trapping reagent, stable isotope labeled glutathione ethyl ester, for the detection of reactive metabolites.
J. Pharmacol. Toxicol. Methods 76 , 83-95, (2015) Glutathione (GSH) trapping assays are widely used to predict the post-marketing risk for idiosyncratic drug reactions (IDRs) in the pharmaceutical industry. Although several GSH derivatives have been ... |
|
|
Antidepressants activate the lysophosphatidic acid receptor LPA(1) to induce insulin-like growth factor-I receptor transactivation, stimulation of ERK1/2 signaling and cell proliferation in CHO-K1 fibroblasts.
Biochem. Pharmacol. 95 , 311-23, (2015) Different lines of evidence indicate that the lysophosphatidic acid (LPA) receptor LPA1 is involved in neurogenesis, synaptic plasticity and anxiety-related behavior, but little is known on whether th... |
|
|
Imaging MALDI MS of Dosed Brain Tissues Utilizing an Alternative Analyte Pre-extraction Approach.
J. Am. Soc. Mass Spectrom. 26 , 967-73, (2015) Matrix-assisted laser desorption ionization (MALDI) imaging mass spectrometry has been adopted in the pharmaceutical industry as a useful tool to detect xenobiotic distribution within tissues. A uniqu... |
| 8-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine |
| Asaleptin |
| 3-chloro-6-(4-methylpiperazin-1-yl)-5H-benzo[b][1,4]benzodiazepine |
| EINECS 227-313-7 |
| 8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[h,e][1,4]diazepine |
| 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine |
| Clorazil |
| 5H-Dibenzo(b,e)(1,4)diazepine, 8-chloro-11-(4-methyl-1-piperazinyl)- |
| Clozapine |
| Clozapine (Tautomer) |
| Iprox |
| 8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine |
| 5H-Dibenzo[b,e][1,4]diazepine, 8-chloro-11-(4-methyl-1-piperazinyl)- |
| Clozapinum |
| Azaleptine |
| Clozaril |
| Clozapin |
| Leponex |
| Fazaclo |
| Lepotex |
| MFCD00153785 |
| 8-CHLORO-11-(4-METHYL-1-PIPERAZINYL)-5H-DIBENZO(B,E)(1,4)DIA - ZEPINE |
![[2-(2-amino-4-chloroanilino)phenyl]-(4-methylpiperazin-1-yl)methanone structure](https://image.chemsrc.com/caspic/085/65514-71-8.png)
![8,11-Dichloro-5H-dibenzo[b,e][1,4]diazepine structure](https://image.chemsrc.com/caspic/283/50373-22-3.png)
![Benzoicacid, 2-[(2-amino-4-chlorophenyl)amino]- structure](https://image.chemsrc.com/caspic/464/67990-66-3.png)
 CAS#:6104-71-8
CAS#:6104-71-8
