2-Chloroadenosine
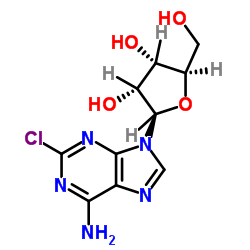
2-Chloroadenosine structure
|
Common Name | 2-Chloroadenosine | ||
|---|---|---|---|---|
| CAS Number | 146-77-0 | Molecular Weight | 301.686 | |
| Density | 2.2±0.1 g/cm3 | Boiling Point | 643.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C10H12ClN5O4 | Melting Point | 162 °C | |
| MSDS | Chinese USA | Flash Point | 342.8±34.3 °C | |
Use of 2-Chloroadenosine2-Chloroadenosine, a stable adenosine analogue, protects against long term development of ischaemic cell loss in the rat hippocampus. 2-Chloroadenosine is an apparent competitive inhibitor of uridine influx (apparent Ki=33 μM) and high-affinity nitrobenzylthioinosine binding (apparent Ki=0.18 mM). 2-Chloroadenosine is a transported permeant for the nucleoside transporter in human erythrocytes[1][2]. |
| Name | 2-Chloroadenosine |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Chloroadenosine, a stable adenosine analogue, protects against long term development of ischaemic cell loss in the rat hippocampus. 2-Chloroadenosine is an apparent competitive inhibitor of uridine influx (apparent Ki=33 μM) and high-affinity nitrobenzylthioinosine binding (apparent Ki=0.18 mM). 2-Chloroadenosine is a transported permeant for the nucleoside transporter in human erythrocytes[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 2.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 643.3±65.0 °C at 760 mmHg |
| Melting Point | 162 °C |
| Molecular Formula | C10H12ClN5O4 |
| Molecular Weight | 301.686 |
| Flash Point | 342.8±34.3 °C |
| Exact Mass | 301.057770 |
| PSA | 139.54000 |
| LogP | -0.46 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.912 |
| Water Solubility | H2O: 10 mg/mL, clear, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AU7357550 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
|
Hypoxanthine uptake by skeletal muscle microvascular endothelial cells from equilibrative nucleoside transporter 1 (ENT1)-null mice: effect of oxidative stress.
Microvasc. Res. 98 , 16-22, (2015) Adenosine is an endogenous regulator of vascular tone. This activity of adenosine is terminated by its uptake and metabolism by microvascular endothelial cells (MVEC). The predominant transporter invo... |
|
|
Oxidative stress modulates nucleobase transport in microvascular endothelial cells
Microvasc. Res. 95 , 68-75, (2014) Purine nucleosides and nucleobases play key roles in the physiological response to vascular ischemia/reperfusion events. The intra- and extracellular concentrations of these compounds are controlled, ... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
| 6-Amino-2-chloro-9-(β-D-ribofuranosyl)purine Hydrate |
| 2-CADO,6-Amino-2-chloropurine riboside |
| 2-Chloroadenosine |
| 6-Amino-2-chloropurine Riboside Hydrate |
| 2-CADO Hydrate |
| EINECS 205-678-3 |
| (2R,3R,4S,5R)-2-(6-Amino-2-chloro-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydro-3,4-furandiol |
| MFCD00005734 |
| Adenosine, 2-chloro- |
| 2-Chlor-9-(β-D-ribofuranosyl)-9H-purin-6-amin |
| 6-Amino-2-Chloropurine Riboside |
| 2-chloro adenosine |
![9-[2,3,5-TRI-O-ACETYL-BETA-D-RIBOFURANOSYL]-2,6-DICHLOROPURINE Structure](https://image.chemsrc.com/caspic/019/3056-18-6.png) CAS#:3056-18-6
CAS#:3056-18-6 CAS#:105499-44-3
CAS#:105499-44-3 CAS#:15465-92-6
CAS#:15465-92-6 CAS#:16321-99-6
CAS#:16321-99-6 CAS#:118-00-3
CAS#:118-00-3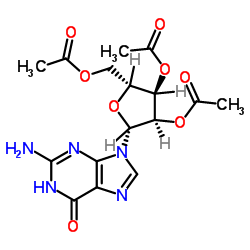 CAS#:6979-94-8
CAS#:6979-94-8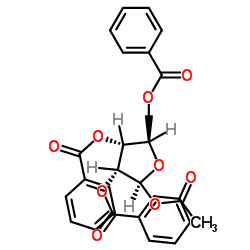 CAS#:6974-32-9
CAS#:6974-32-9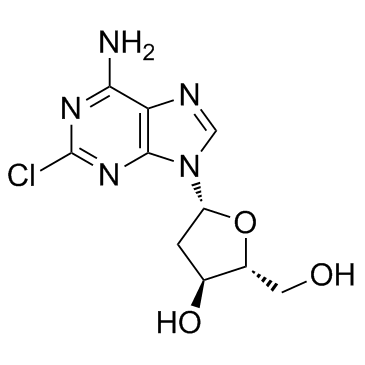 CAS#:4291-63-8
CAS#:4291-63-8 CAS#:43157-50-2
CAS#:43157-50-2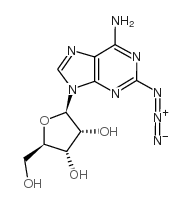 CAS#:59587-07-4
CAS#:59587-07-4![2-Chloro-2',3',5'-tris-O-[(1,1-dimethylethyl)dimethylsilyl]-adenosine structure](https://image.chemsrc.com/caspic/254/195727-26-5.png) CAS#:195727-26-5
CAS#:195727-26-5 CAS#:58-61-7
CAS#:58-61-7 CAS#:28783-37-1
CAS#:28783-37-1![2-[2-(4-bromo-2-thienyl)-ethylamino]-adenosine structure](https://image.chemsrc.com/caspic/211/124498-79-9.png) CAS#:124498-79-9
CAS#:124498-79-9 CAS#:24639-06-3
CAS#:24639-06-3 CAS#:13364-95-9
CAS#:13364-95-9
