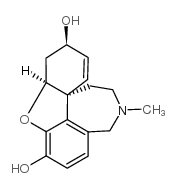
357-70-0
| Name | galanthamine |
|---|---|
| Synonyms |
MFCD00867189
3,4-Dihydrogalanthamine Galantamine Nivalin Lycoremine Galantamine Hydrobromide (4aS,6R,8aS)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2]benzazepin-6-ol Galanthamine |
| Description | Galanthamine is a potent acetylcholinesterase (AChE) inhibitor with an IC50 of 500 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.5 μM (AChE)[1] |
| In Vitro | Galanthamine inhibits AChE and BChE with IC50 of 0.5 and 8.5 μM[1]. Galanthamine acts as a positive allosteric modulator (PAM) of human α4β2 AChRs expressed in permanently transfected HEK 293 cells. Galanthamine increases the response of (α4β2)2α5 AChRs to 1 μM ACh by up to 220% with very low concerntration(EC50=0.25 nM). Only small potentiation (20%) of either α4β2 or (α4β2)2β3 AChRs is detected using FLEXstation assays. Galanthamine at concentrations of 1 μM and above inhibits all three AChR subtypes[2]. |
| In Vivo | Acute administration of Galantamine (0.3-3 mg/kg, i.p.) increases IGF2 mRNA levels in the hippocampus, but not in the prefrontal cortex, in time- and dose-dependent manner. Galantamine (3 mg/kg, i.p.) causes a transient increase in fibroblast growth factor 2 mRNA levels and a decrease in brain-derived neurotrophic factor mRNA levels in the hippocampus, while it does not affect the mRNA levels of other neurotrophic/growth factors. The Galantamine-induced increase in the hippocampal IGF2 mRNA levels is blocked by Mecamylamine, a nonselective nicotinic acetylcholine (ACh) receptor (nAChR) antagonist, and Methyllycaconitine, a selective α7 nAChR antagonist, but not by Telenzepine, a preferential M1muscarinic ACh receptor antagonist. Moreover, the selective α7 nAChR agonist PHA-543613 increasea the IGF2 mRNA levels, while Donepezil, an acetylcholinesterase inhibitor, does not. Galantamine also increases hippocampal IGF2 protein, which is blocked by Methyllycaconitine[2]. |
| Animal Admin | Mice[2] Eight-week-old male ddY mice are housed in cages (24 cm×17 cm×12 cm) in each group of five to six animals under controlled environmental conditions (22±1°C; 12:12-h light-dark cycle, lights on at 0800 hours, food and water ad libitum) for 1 week before use in the experiments. 453 mice are used in total and in single use for each purpose. The following drugs are used: mecamylamine, methyllycaconitine, oxotremorine, and telenzepine, and Galantamine, Donepezil, and PHA-543613. All drugs are dissolved in saline (0.9 % solution of NaCl). Drugs are administered in a volume of 10 mL/kg intraperitoneally (i.p.) (Galantamine, Donepezil, Mecamylamine, Methyllycaconitine, Oxotremorine) or subcutaneously (s.c.) (PHA-543613, Telenzepine). |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 439.3±45.0 °C at 760 mmHg |
| Melting Point | 119-121ºC |
| Molecular Formula | C17H21NO3 |
| Molecular Weight | 287.353 |
| Flash Point | 219.5±28.7 °C |
| Exact Mass | 287.152130 |
| PSA | 41.93000 |
| LogP | 1.75 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.636 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| HS Code | 2942000000 |
| Precursor 10 | |
|---|---|
| DownStream 7 | |
| HS Code | 2942000000 |
|---|
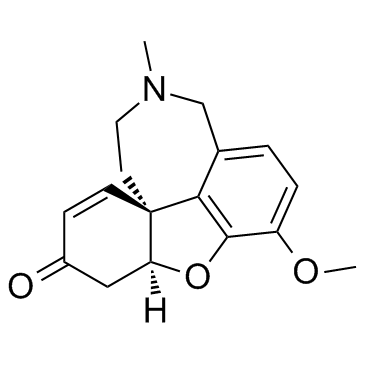
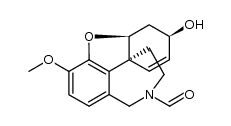
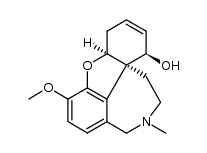
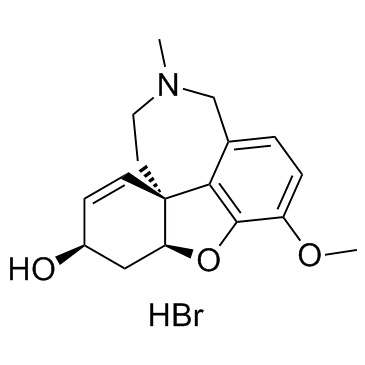
![(4aR,6S,8aR)-4a,5,11,12-tetrahydro-6-hydroxy-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2]-benzazepin-10(9H)-one structure](https://image.chemsrc.com/caspic/019/869369-22-2.png)
![(2S,5R)-5-benzyl-2-(3,5-dibenzyloxy-4-methoxyphenyl)-3-[2-(4-hydroxyphenyl)ethyl]imidazolidin-4-one structure](https://image.chemsrc.com/caspic/029/722499-71-0.png)
![(4aS,8aS,13R,14aR)-13-benzyl-2-hydroxy-3-methoxy-14-(2,2,2-trifluoroacetyl)-4a,5,9,10,14,14a-hexahydro-6H-benzo[2,3]benzofuro[4,3-cd]imidazo[1,2-a]azepine-6,12(13H)-dione structure](https://image.chemsrc.com/caspic/415/722499-74-3.png)
![(4aS,8aS,13R,14aR)-13-benzyl-3-methoxy-6,12-dioxo-14-(2,2,2-trifluoroacetyl)-4a,5,9,10,12,13,14,14a-octahydro-6H-benzo[2,3]benzofuro[4,3-cd]imidazo[1,2-a]azepin-2-yl trifluoromethanesulfonate structure](https://image.chemsrc.com/caspic/360/722499-80-1.png)
![(2R,11bR)-2-benzyl-8,10-bis(benzyloxy)-9-methoxy-1-(2,2,2-trifluoroacetyl)-1,5,6,11b-tetrahydrospiro[benzo[c]imidazo[1,2-a]azepine-7,1'-cyclohexane]-2',5'-diene-3,4'(2H)-dione structure](https://image.chemsrc.com/caspic/453/722499-73-2.png)
![(2S,5R)-5-benzyl-2-(3,5-dibenzyloxy-4-methoxyphenyl)-3-[2-(4-hydroxyphenyl)ethyl]-1-(2,2,2-trifluoroacetyl)imidazolidin-4-one structure](https://image.chemsrc.com/caspic/491/722499-72-1.png)