| Structure | Name/CAS No. | Articles |
|---|---|---|
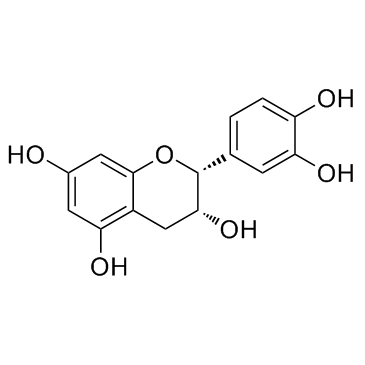 |
Epicatechin
CAS:490-46-0 |
|
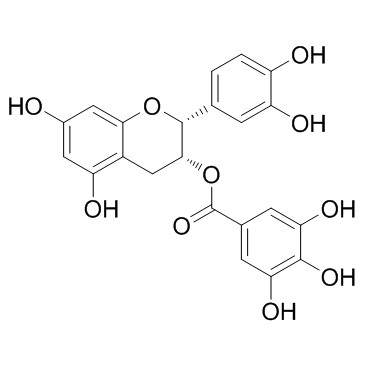 |
(-)-Epicatechin gallate
CAS:1257-08-5 |
|
 |
(-)-Epigallocatechin(EGC)
CAS:970-74-1 |
|
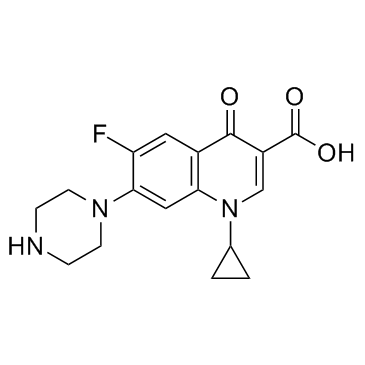 |
Ciprofloxacin
CAS:85721-33-1 |
|
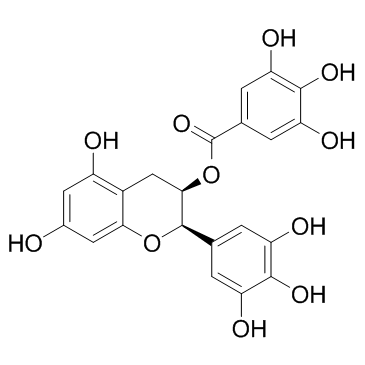 |
(-)-Epigallocatechin gallate
CAS:989-51-5 |