| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
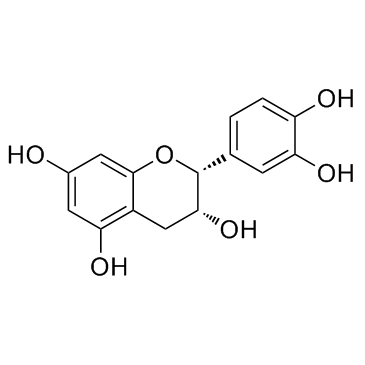 |
表儿茶素(EC)
CAS:490-46-0 |
|
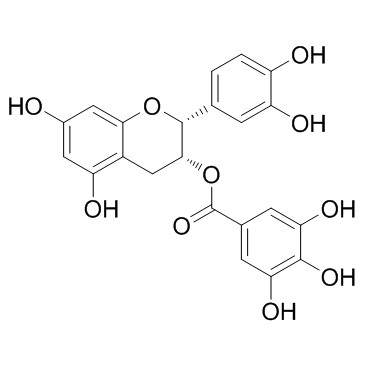 |
表儿茶素没食子酸酯
CAS:1257-08-5 |
|
 |
表没食子儿茶素
CAS:970-74-1 |
|
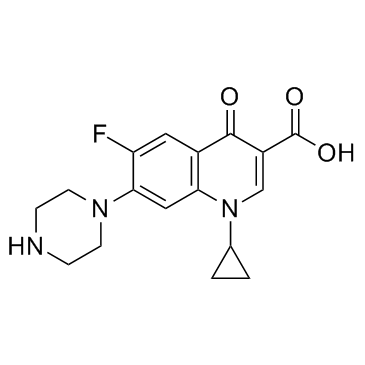 |
环丙沙星
CAS:85721-33-1 |
|
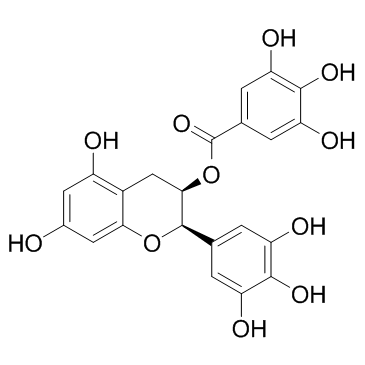 |
(-)-表没食子儿茶素没食子酸酯
CAS:989-51-5 |