CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
VB1993800
-
CHEMICAL NAME :
-
3-Quinolinecarboxylic acid, 1,4-dihydro-1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperaz inyl)-
-
CAS REGISTRY NUMBER :
-
85721-33-1
-
LAST UPDATED :
-
199709
-
DATA ITEMS CITED :
-
29
-
MOLECULAR FORMULA :
-
C17-H18-F-N3-O3
-
MOLECULAR WEIGHT :
-
331.38
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
21429 ug/kg/3D-I
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - toxic psychosis
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
21 mg/kg/3D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume decreased Kidney, Ureter, Bladder - proteinuria Kidney, Ureter, Bladder - hematuria
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
5714 ug/kg/2D-I
-
TOXIC EFFECTS :
-
Behavioral - coma Liver - jaundice, other or unclassified Liver - liver function tests impaired
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
129 mg/kg/6D-I
-
TOXIC EFFECTS :
-
Behavioral - rigidity (including catalepsy)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
135 mg/kg/3D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
40 mg/kg/2D-I
-
TOXIC EFFECTS :
-
Behavioral - headache Blood - eosinophilia Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Multiple routes
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
40 mg/kg/6D-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, allergic (after systemic exposure) Skin and Appendages - dermatitis, other (after systemic exposure)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
207 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1165 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
122 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity)
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Sister chromatid exchange
MUTATION DATA
-
TYPE OF TEST :
-
DNA inhibition
-
TEST SYSTEM :
-
Mammal - domestic Cells - not otherwise specified
-
DOSE/DURATION :
-
27 mg/L
-
REFERENCE :
-
AMACCQ Antimicrobial Agents and Chemotherapy. (American Soc. for Microbiology, 1913 I St., NW, Washington, DC 20006) V.1- 1972- Volume(issue)/page/year: 34,1955,1990
|
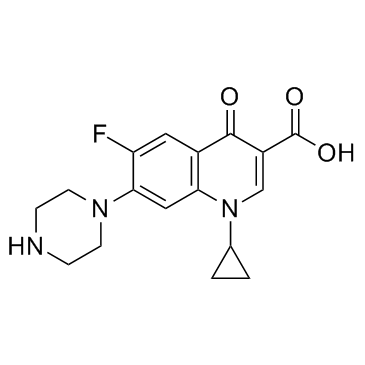
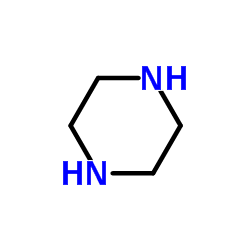 CAS#:110-85-0
CAS#:110-85-0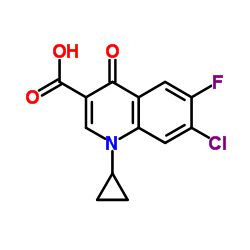 CAS#:86393-33-1
CAS#:86393-33-1 CAS#:67-68-5
CAS#:67-68-5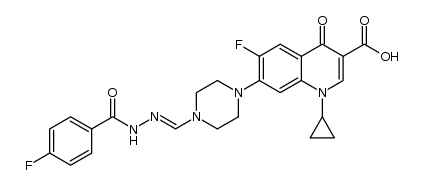 CAS#:1340587-05-4
CAS#:1340587-05-4 CAS#:88419-56-1
CAS#:88419-56-1 CAS#:121872-95-5
CAS#:121872-95-5 CAS#:54393-21-4
CAS#:54393-21-4 CAS#:765-30-0
CAS#:765-30-0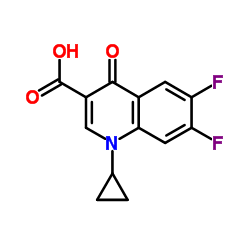 CAS#:93107-30-3
CAS#:93107-30-3![3-Quinolinecarboxylic acid, 1-cyclopropyl-7-[4-(ethoxycarbonyl)-1-piperazinyl]-6-fluoro-1,4-dihydro-4-oxo Structure](https://image.chemsrc.com/caspic/288/93594-29-7.png) CAS#:93594-29-7
CAS#:93594-29-7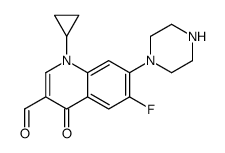 CAS#:110719-57-8
CAS#:110719-57-8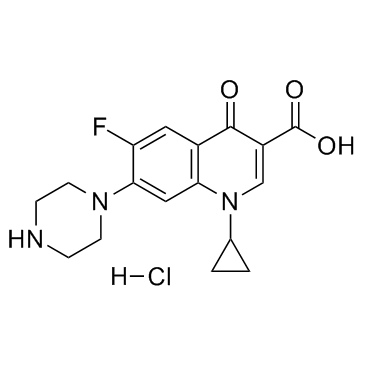 CAS#:93107-08-5
CAS#:93107-08-5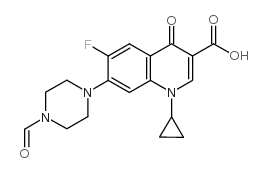 CAS#:93594-39-9
CAS#:93594-39-9 CAS#:105394-83-0
CAS#:105394-83-0 CAS#:93107-11-0
CAS#:93107-11-0 CAS#:952653-63-3
CAS#:952653-63-3
