(-)-Epigallocatechin gallate
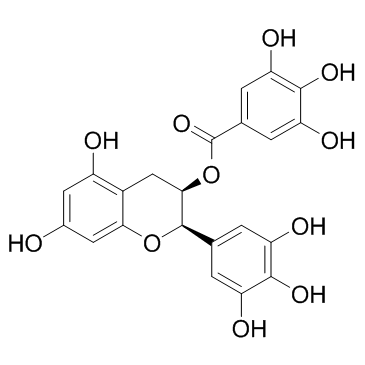
(-)-Epigallocatechin gallate structure
|
Common Name | (-)-Epigallocatechin gallate | ||
|---|---|---|---|---|
| CAS Number | 989-51-5 | Molecular Weight | 458.372 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 909.1±65.0 °C at 760 mmHg | |
| Molecular Formula | C22H18O11 | Melting Point | 222-224°C | |
| MSDS | Chinese USA | Flash Point | 320.0±27.8 °C | |
Use of (-)-Epigallocatechin gallate(-)-Epigallocatechin Gallate is an antioxidant polyphenol flavonoid form green tea, and inhibits the activation of EGFR, HER2 and HER3, with antitumor activity. |
| Name | (-)-epigallocatechin 3-gallate |
|---|---|
| Synonym | More Synonyms |
| Description | (-)-Epigallocatechin Gallate is an antioxidant polyphenol flavonoid form green tea, and inhibits the activation of EGFR, HER2 and HER3, with antitumor activity. |
|---|---|
| Related Catalog | |
| Target |
EGFR HER2 HER3 |
| In Vitro | (-)-Epigallocatechin Gallate (EGCG) inhibits activation EGFR, HER2 and HER3 in the SW837 human colon cancer cell line. (-)-Epigallocatechin Gallate (10 μM) also inhibits cell growth, suppresses activation of EGFR, HER2, and HER3, and causes decrease in the levels of COX-2 and Bcl-xL proteins, and apoptosis after treatment for 96 h[1]. (-)-Epigallocatechin Gallate (0-35 μg/mL) inhibits the proliferation of colorectal cancer cells. (-)-Epigallocatechin Gallate (35 μg/mL) induces apoptosis of colorectal cancer cells[2]. (-)-Epigallocatechin Gallate (EGCG; 50, 75 and 100 μM) dose-dependently inhibits the growth of HepG2 cells, and induces apoptosis in HepG2 cells[3]. |
| In Vivo | (-)-Epigallocatechin Gallate (5, 10, and 20 mg/kg, p.o.) inhibits the growth of orthotopic colorectal cancer cells in mice[2]. |
| Cell Assay | LoVo, SW480, HCT-8, and HT-29 cells are seeded in 96-well plates at a concentration of 5×103 cells; each cell line is totally seeded in the 12 wells. Complete medium is added to the wells, up to 200 μL; the medium contains 0 μg/mL, 10 μg/mL, 20 μg/mL, and 35 μg/mL of (-)-Epigallocatechin Gallate. The inhibition rate=[1 - (absorbance of (-)-Epigallocatechin Gallate group - absorbance of control group)/(absorbance of control group - absorbance of blank control group)] × 100[2]. |
| Animal Admin | Mice[2] At 2 weeks postsurgery, 39 out of the 40 nude mice presented with tumors. Based on the volume of the tumors, the 39 mice with tumors are divided into four groups: a control group (n=9); a group that receives 5 mg/kg of (-)-Epigallocatechin Gallate (n=10); a group that receives 10 mg/kg of (-)-Epigallocatechin Gallate (n=10); and a group that receives 20 mg/kg of (-)-Epigallocatechin Gallate (n=10). In the therapeutic groups, (-)-Epigallocatechin Gallate is administrated intragastrically, and in the control group, 100 uL of physiological saline is administrated intragastrically, once daily for 14 days. After the treatment of the mice with (-)-Epigallocatechin Gallate for 4 weeks, the growth and metastasis of the primary tumors are continuously monitored using a fluorescent imaging system. After 4 weeks, the primary tumors are weighed and immediately put into liquid nitrogen (−196°C) and 2 to 3 hours later, these specimens are stored at −80°C. In addition, the other parts of the primary tumor and metastases are fixed in 4% formaldehyde[2]. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 909.1±65.0 °C at 760 mmHg |
| Melting Point | 222-224°C |
| Molecular Formula | C22H18O11 |
| Molecular Weight | 458.372 |
| Flash Point | 320.0±27.8 °C |
| Exact Mass | 458.084900 |
| PSA | 197.37000 |
| LogP | 2.08 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.857 |
| Storage condition | 2-8°C |
| Stability | Stable, but may be light sensitive. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | F+ |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | KB5200000 |
| Precursor 8 | |
|---|---|
| DownStream 9 | |
|
Mechanism of Dose-Dependent Regulation of UBE1L by Polyphenols in Human Bronchial Epithelial Cells.
J. Cell. Biochem. 116 , 1553-62, (2015) Ubiquitin activating enzyme E1-like (UBE1L) is the activating enzyme for ISG15ylation (ISG15, interferon stimulated gene 15). UBE1L is thought to be a candidate tumor suppressor gene and has positive ... |
|
|
Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway.
Toxicol. Appl. Pharmacol. 283(1) , 65-74, (2015) (-)-Epigallocatechin-3-gallate (EGCG), a constituent of green tea, has been suggested to have numerous health-promoting effects. On the other hand, high-dose EGCG is able to evoke hepatotoxicity. In t... |
|
|
Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells.
Mol. Carcinog. 54(6) , 485-99, (2015) Aberrant epigenetic silencing of the tissue inhibitor of matrix metalloproteinase-3 (TIMP-3) gene that negatively regulates matrix metalloproteinases (MMPs) activity has been implicated in the pathoge... |
| TEA CATECHIN |
| (-)-epigallocatechin 3-gallate |
| (2R,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl 3,4,5-trihydroxybenzoate |
| Epigallocatechin 3-gallate |
| EGCG |
| MFCD00075940 |
| Benzoic acid, 3,4,5-trihydroxy-, (2R,3R)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester |
| (-)-Epigallocatechin-3-o-gallate |
| Epigallocatechin gallate |
| E-5187 |
| (-)-Epigallocatechin 3-O-gallate |
| 3,4,5-Trihydroxybenzoic acid (2R-cis)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester |
| (-)-EGCG |
| Teavigo |
| ECGC |
| EINECS 479-560-7 |
| Teavigo TG |
| (-)-Epigallocatechin gallate |
| EGCG-d6 |
| Epigallocatechin-3-gallate |
| (−)-cis-3,3',4',5,5',7-Hexahydroxy-flavane-3-gallate |
![(-)-(2R,3R)-cis-5,7-bis(benzyloxy)-2-[3,4,5-tris(benzyloxy)phenyl]chroman-3-yl 3,4,5-tris(benzyloxy)benzoate Structure](https://image.chemsrc.com/caspic/464/332386-77-3.png) CAS#:332386-77-3
CAS#:332386-77-3 CAS#:1486-48-2
CAS#:1486-48-2 CAS#:1486-47-1
CAS#:1486-47-1![(-)-(2R,3R)-cis-5,7-bis(benzyloxy)-2-[3,4,5-tris(benzyloxy)phenyl]chroman-3-ol Structure](https://image.chemsrc.com/caspic/078/332386-74-0.png) CAS#:332386-74-0
CAS#:332386-74-0![(-)-(2R,3S)-trans-5,7-bis(benzyloxy)-2-[3,4,5-tris(benzyloxy)phenyl]chroman-3-ol Structure](https://image.chemsrc.com/caspic/202/332386-71-7.png) CAS#:332386-71-7
CAS#:332386-71-7 CAS#:121795-67-3
CAS#:121795-67-3 CAS#:121795-66-2
CAS#:121795-66-2 CAS#:121844-27-7
CAS#:121844-27-7 CAS#:1707-75-1
CAS#:1707-75-1 CAS#:149-91-7
CAS#:149-91-7 CAS#:490-46-0
CAS#:490-46-0 CAS#:970-74-1
CAS#:970-74-1 CAS#:569-77-7
CAS#:569-77-7![[5,7-dihydroxy-2-(2,3,4,5-tetrahydroxy-6-oxobenzo[7]annulen-8-yl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate structure](https://image.chemsrc.com/caspic/056/102067-92-5.png) CAS#:102067-92-5
CAS#:102067-92-5 CAS#:30462-35-2
CAS#:30462-35-2 CAS#:30462-34-1
CAS#:30462-34-1
