Thiocolchicine
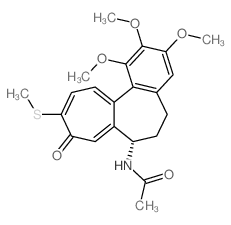
Thiocolchicine structure
|
Common Name | Thiocolchicine | ||
|---|---|---|---|---|
| CAS Number | 2730-71-4 | Molecular Weight | 415.50300 | |
| Density | 1.27g/cm3 | Boiling Point | 729.1ºC at 760mmHg | |
| Molecular Formula | C22H25NO5S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 394.7ºC | |
| Symbol |



GHS05, GHS06, GHS08 |
Signal Word | Danger | |
|
Antiproliferative activity of colchicine analogues on MDR-positive and MDR-negative human cancer cell lines.
Anticancer Drug Des. 13 , 19-33, (1998) In this study the in vitro antitumor activity of a series of 20 colchicine analogues was tested and compared with colchicine and thiocolchicine on three different human cancer cell lines, two of which express the multidrug-resistance (MDR) phenotype. At conce... |
|
|
Antitumor agents--CLXXV. Anti-tubulin action of (+)-thiocolchicine prepared by partial synthesis.
Bioorg. Med. Chem. 5(12) , 2277-82, (1997) (+)-Thiocolchicine (2b) was prepared from (+/-)-colchicine (1) in a five-step reaction sequence that included chromatographic separation of appropriate camphanylated diastereomers. Acid hydrolysis of the (+)-diastereomer, followed by acetylation, yielded the ... |
|
|
Thiocolchicine dimers: a novel class of topoisomerase-I inhibitors.
Biochem. Pharmacol. 69(1) , 113-21, (2005) During a cellular screening of thiocolchicine analogs, thiocolchicine dimers resulted particularly active in cisplatin-resistant A2780-CIS cells. In order to discover by which mechanism(s) thiocolchicine dimers overcame cisplatin resistance, p53, p21waf1 and ... |
|
|
Derivatives of thiocolchicine and its applications to CEM cells treatment using 19F magnetic resonance ex vivo.
Bioorg. Chem. 38(1) , 1-6, (2010) It was shown, that cultured ex vivo human T-Lymphoblastoid (CEM) cells respond to synthesized thiocolchicine and fluorine thiocolchicine derivatives. The preparation of derivatives with substitution at C-3 and C-7 is described. All compounds were used at conc... |
|
|
Association of thiocolchicine with tubulin.
Biochem. Biophys. Res. Commun. 161(2) , 544-50, (1989) Thiocolchicine, a colchicine analog in which the C-10 methoxy is replaced with a thiomethyl moiety, was shown to bind with high affinity to the colchicine site on tubulin (Ka = 1.07 +/- 0.14 x 10(6) M-1 at 23 degrees C). Like colchicine, the association kinet... |
|
|
Comparative pharmacokinetics and bioavailability of two oral formulations of thiocolchicoside, a GABA-mimetic muscle relaxant drug, in normal volunteers.
Eur. J. Drug Metab. Pharmacokinet. 20(4) , 301-5, (1995) The comparative pharmacokinetic and bioavailability profile of two different formulations (tablets and capsules) of thiocolchicoside was investigated in 8 healthy male volunteers after administration of single oral 8 mg doses. Plasma samples were assayed by a... |
|
|
Biological evaluation on different human cancer cell lines of novel colchicine analogs.
Oncol. Res. 11(3) , 145-52, (1999) Three new 7-0-substituted deacetamidothiocolchicine derivatives have been evaluated for their antitumor activity against various human tumor cell lines, some of which express the multidrug resistance (MDR) phenotype, for their impact on the cell cycle and the... |
|
|
New synthetic thiocolchicine derivatives as lowtoxic anticancer agents.
Arch. Pharm. (Weinheim) 338(12) , 582-9, (2005) New thiocolchicine derivatives (1-8) were designed as less toxic anticancer agents possessing the power full anticancer activity of colchicine. The synthesis and biological evaluation of these compounds were described. As a preliminary result of biological in... |
|
|
Interaction of novel thiocolchicine analogs with the tubulin isoforms from bovine brain.
Biochem. Biophys. Res. Commun. 254(2) , 334-7, (1999) The antimitotic alkaloid colchicine binds to tubulin and inhibits microtubule assembly. Recently a new series of colchicine derivatives has been synthesized in which the seven-membered B-ring was shortened to a six-membered ring. In an effort to study the rol... |
|
|
[TENS + mesotherapy association in the therapy of cervico-brachialgia: preliminary data].
Minerva Anestesiol. 57(10) , 1084-5, (1991)
|