Thiocolchicine
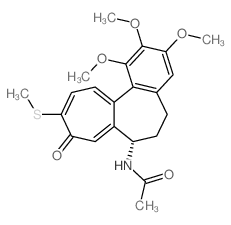
Thiocolchicine structure
|
Common Name | Thiocolchicine | ||
|---|---|---|---|---|
| CAS Number | 2730-71-4 | Molecular Weight | 415.50300 | |
| Density | 1.27g/cm3 | Boiling Point | 729.1ºC at 760mmHg | |
| Molecular Formula | C22H25NO5S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 394.7ºC | |
| Symbol |



GHS05, GHS06, GHS08 |
Signal Word | Danger | |
Use of ThiocolchicineThiocolchicine, a derivative modified in the C Ring of Colchicine (HY-16569) with enhanced biological properties. Thiocolchicine is a potent inhibitor of tubulin polymerization (IC50=2.5 µM) and competitively binds to tubulin with a Ki of 0.7 µM. Thiocolchicine induces cell apoptosis[1][2]. Thiocolchicine can be used as an ADC cytotoxin in ADC technology. |
| Name | N-[(7S)-1,2,3-trimethoxy-10-methylsulfanyl-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | Thiocolchicine, a derivative modified in the C Ring of Colchicine (HY-16569) with enhanced biological properties. Thiocolchicine is a potent inhibitor of tubulin polymerization (IC50=2.5 µM) and competitively binds to tubulin with a Ki of 0.7 µM. Thiocolchicine induces cell apoptosis[1][2]. Thiocolchicine can be used as an ADC cytotoxin in ADC technology. |
|---|---|
| Related Catalog | |
| In Vitro | Thiocolchicine is against MCF-7, LoVo, LoVo/DX, A-549 and BALB/3T3 cells with IC50 values of 0.01 μM, 0.021 μM, 0.398 μM, 0.011 μM and 0.114 μM, respectively[3]. Thiocolchicine (1 nM-100 μM; 24-72 hours) shows a relationship between cell cycle blocking activity and growth inhibition in breast cancer cells. It inhibits cell proliferation of MDA-MB-231 and multidrug resistant (MDR) MCF-7 ADRr breast cancer cells with IC50s of 0.6 nM and 400 nM, respectively, as well as MDR CEM-VBL leukemia cells (IC50=50 nM)[2]. |
| References |
| Density | 1.27g/cm3 |
|---|---|
| Boiling Point | 729.1ºC at 760mmHg |
| Molecular Formula | C22H25NO5S |
| Molecular Weight | 415.50300 |
| Flash Point | 394.7ºC |
| Exact Mass | 415.14500 |
| PSA | 99.16000 |
| LogP | 3.97580 |
| Vapour Pressure | 4.12E-21mmHg at 25°C |
| Index of Refraction | 1.609 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS05, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 + H330-H318-H340 |
| Precautionary Statements | P201-P260-P264-P280-P284-P301 + P310 |
| Hazard Codes | T+ |
| RIDADR | UN 1544PSN1 6.1 / PGI |
| Precursor 7 | |
|---|---|
| DownStream 7 | |
|
Antiproliferative activity of colchicine analogues on MDR-positive and MDR-negative human cancer cell lines.
Anticancer Drug Des. 13 , 19-33, (1998) In this study the in vitro antitumor activity of a series of 20 colchicine analogues was tested and compared with colchicine and thiocolchicine on three different human cancer cell lines, two of which... |
|
|
Antitumor agents--CLXXV. Anti-tubulin action of (+)-thiocolchicine prepared by partial synthesis.
Bioorg. Med. Chem. 5(12) , 2277-82, (1997) (+)-Thiocolchicine (2b) was prepared from (+/-)-colchicine (1) in a five-step reaction sequence that included chromatographic separation of appropriate camphanylated diastereomers. Acid hydrolysis of ... |
|
|
Thiocolchicine dimers: a novel class of topoisomerase-I inhibitors.
Biochem. Pharmacol. 69(1) , 113-21, (2005) During a cellular screening of thiocolchicine analogs, thiocolchicine dimers resulted particularly active in cisplatin-resistant A2780-CIS cells. In order to discover by which mechanism(s) thiocolchic... |
| thiocolhicine |
| N-[(7S)-1,2,3-trimethoxy-10-methylsulfanyl-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide |
| 10-Demethoxy-10-methylthiocolchicine |
| N-((S)-1,2,3-trimethoxy-10-methylsulfanyl-9-oxo-5,6,7,9-tetrahydro-benzo[a]heptalen-7-yl)-acetamide |
| Thiocolchicine |
| thiocolchicine |
| EINECS 220-346-8 |
| Colchicine,10-thio |
| N-((S)-1,2,3-Trimethoxy-10-methylmercapto-9-oxo-5,6,7,9-tetrahydro-benzo[a]heptalen-7-yl)-acetamid |
| Thiocholchicine |
| Colchicine,10-demethoxy-10-(methylthio) |
 CAS#:64-86-8
CAS#:64-86-8 CAS#:5188-07-8
CAS#:5188-07-8 CAS#:74-93-1
CAS#:74-93-1![Benzo[a]heptalen-9(5H)-one,7-amino-6,7-dihydro-1,2,3-trimethoxy-10-(methylthio)-, (7S)- Structure](https://image.chemsrc.com/caspic/394/2731-16-0.png) CAS#:2731-16-0
CAS#:2731-16-0 CAS#:108-24-7
CAS#:108-24-7![N-(1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl)acetamide Structure](https://image.chemsrc.com/caspic/493/209810-38-8.png) CAS#:209810-38-8
CAS#:209810-38-8 CAS#:186581-53-3
CAS#:186581-53-3![Acetamide,N-[(7S)-5,6,7,9-tetrahydro-1,2,3-trimethoxy-10-(methylsulfonyl)-9-oxobenzo[a]heptalen-7-yl]- structure](https://image.chemsrc.com/caspic/095/2826-75-7.png) CAS#:2826-75-7
CAS#:2826-75-7 CAS#:87424-25-7
CAS#:87424-25-7![N-(1,2,3-trimethoxy-10-methylsulfinyl-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl)acetamide structure](https://image.chemsrc.com/caspic/178/76189-03-2.png) CAS#:76189-03-2
CAS#:76189-03-2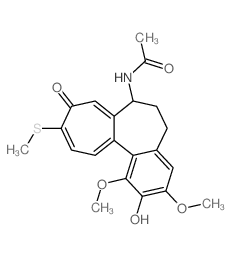 CAS#:87424-26-8
CAS#:87424-26-8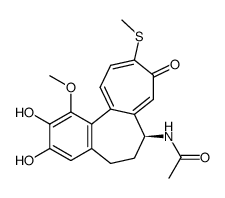 CAS#:51296-12-9
CAS#:51296-12-9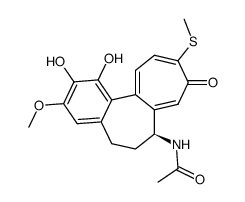 CAS#:51296-11-8
CAS#:51296-11-8