Derivatives of thiocolchicine and its applications to CEM cells treatment using 19F magnetic resonance ex vivo.
Dorota Bartusik, Boguslaw Tomanek, Erika Lattová, Hélène Perreault, Jack Tuszynski, Gino Fallone
Index: Bioorg. Chem. 38(1) , 1-6, (2010)
Full Text: HTML
Abstract
It was shown, that cultured ex vivo human T-Lymphoblastoid (CEM) cells respond to synthesized thiocolchicine and fluorine thiocolchicine derivatives. The preparation of derivatives with substitution at C-3 and C-7 is described. All compounds were used at concentration from 1 nM to 1000 nM. Inhibitory effects of these compounds were examined in the three-dimensional (3-D) culture and cells morphology during treatment was monitored using 9.4 T MRI system. We performed studies of these compounds in CEM cells ex vivo using 1H and 19F Magnetic Resonance Imaging (MRI), 19F Magnetic Resonance Spectroscopy (MRS), High Performance Liquid Chromatography coupled with Ultra Violet (HPLC-UV) and Electron Impact Mass Spectrometry (EIMS). The results of the multi-technique approach are consistent with the fact that the new derivatives are more efficient than colchicine and thiocolchicine ex vivo.Crown Copyright 2009. Published by Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
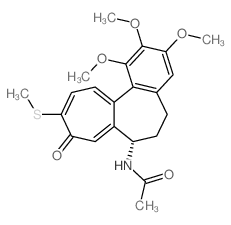 |
Thiocolchicine
CAS:2730-71-4 |
C22H25NO5S |
|
Antiproliferative activity of colchicine analogues on MDR-po...
1998-01-01 [Anticancer Drug Des. 13 , 19-33, (1998)] |
|
Antitumor agents--CLXXV. Anti-tubulin action of (+)-thiocolc...
1997-12-01 [Bioorg. Med. Chem. 5(12) , 2277-82, (1997)] |
|
Thiocolchicine dimers: a novel class of topoisomerase-I inhi...
2005-01-01 [Biochem. Pharmacol. 69(1) , 113-21, (2005)] |
|
Association of thiocolchicine with tubulin.
1989-06-15 [Biochem. Biophys. Res. Commun. 161(2) , 544-50, (1989)] |
|
Comparative pharmacokinetics and bioavailability of two oral...
1995-01-01 [Eur. J. Drug Metab. Pharmacokinet. 20(4) , 301-5, (1995)] |