4-Hydroxyacetophenone
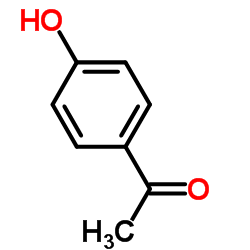
4-Hydroxyacetophenone structure
|
Common Name | 4-Hydroxyacetophenone | ||
|---|---|---|---|---|
| CAS Number | 99-93-4 | Molecular Weight | 136.148 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 313.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H8O2 | Melting Point | 132-135 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 121.2±12.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Convenient QSAR model for predicting the complexation of structurally diverse compounds with β-cyclodextrins
Bioorg. Med. Chem. 17 , 896-904, (2009) This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoretical molecular descriptors, calculated solely from the mole... |
|
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a quantitative structure-activity relationship study.
J. Med. Chem. 48 , 7234-42, (2005) In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop ... |
|
|
Development of an analytical method for the targeted screening and multi-residue quantification of environmental contaminants in urine by liquid chromatography coupled to high resolution mass spectrometry for evaluation of human exposures.
Talanta 146 , 694-706, (2015) The aim of this study was to develop an analytical method and contribute to the assessment of the Exposome. Thus, a targeted analysis of a wide range of contaminants in contact with humans on daily routines in urine was developed. The method focused on a list... |
|
|
Improved sensitivity gas chromatography-mass spectrometry determination of parabens in waters using ionic liquids.
Talanta 146 , 568-74, (2015) A new procedure for the introduction of ionic liquid samples in gas chromatography (GC) is proposed. This procedure, based on microvial insert thermal desorption, allows the direct analysis of the compounds preconcentrated by ionic liquid based liquid-liquid ... |
|
|
Inhibition of GABA shunt enzymes' activity by 4-hydroxybenzaldehyde derivatives.
Bioorg. Med. Chem. Lett. 16 , 592-5, (2006) 4-Hydroxybenzaldehyde (HBA) derivatives were examined as inhibitors for GABA transaminase (GABA-T) and succinic semialdehyde dehydrogenase (SSADH). Investigation of structure-activity relation revealed that a carbonyl group or an amino group as well as a hydr... |
|
|
Phenolic constituents of the Chilean herbal tea Fabiana imbricata R. et P.
Plant Foods Hum. Nutr. 67(3) , 242-6, (2012) "Pichi" or "pichi romero" (Fabiana imbricata R. et. P., Solanaceae) is a Chilean plant used as a tea in the Andean regions of Chile and Argentina. A very simple and direct method was developed for the qualitative analysis of polyphenols in the tea by high-per... |
|
|
Structural characterization and function determination of a nonspecific carboxylate esterase from the amidohydrolase superfamily with a promiscuous ability to hydrolyze methylphosphonate esters.
Biochemistry 53(21) , 3476-85, (2014) The uncharacterized protein Rsp3690 from Rhodobacter sphaeroides is a member of the amidohydrolase superfamily of enzymes. In this investigation the gene for Rsp3690 was expressed in Escherichia coli and purified to homogeneity, and the three-dimensional stru... |
|
|
Select acetophenones modulate flagellar motility in chlamydomonas.
Chem. Biol. Drug Des. 75 , 333-337, (2010) Acetophenones were screened for activity against positive phototaxis of Chlamydomonas cells, a process that requires co-ordinated flagellar motility. The structure-activity relationships of a series of acetophenones are reported, including acetophenones that ... |
|
|
Synthesis, biochemical evaluation and rationalisation of the inhibitory activity of a series of 4-hydroxyphenyl ketones as potential inhibitors of 17beta-hydroxysteroid dehydrogenase type 3 (17beta-HSD3).
Bioorg. Med. Chem. Lett. 16 , 4519-22, (2006) We report the preliminary results of the synthesis and biochemical evaluation of a number of 4-hydroxyphenyl ketones as inhibitors of the isozyme of the enzyme 17beta-hydroxysteroid dehydrogenase (17beta-HSD) responsible for the conversion of androstenedione ... |
|
|
Time-resolved resonance Raman and density functional theory study of the deprotonation reaction of the triplet state of p-hydroxyacetophenone in water solution.
J. Org. Chem. 70(22) , 8661-75, (2005) [reaction; see text] Picosecond and nanosecond time-resolved resonance Raman (TR(3)) spectroscopy was employed to investigate the deprotonation/ionization reaction of p-hydroxyacetophenone (HA) after ultraviolet photolysis in water solution. The TR(3) spectra... |