| Structure | Name/CAS No. | Articles |
|---|---|---|
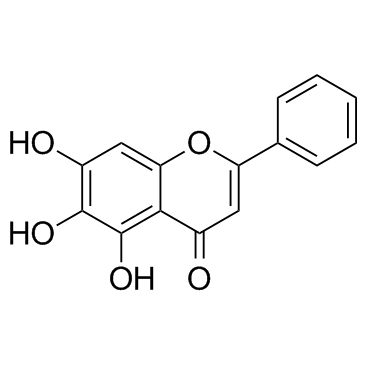 |
Baicalein
CAS:491-67-8 |
|
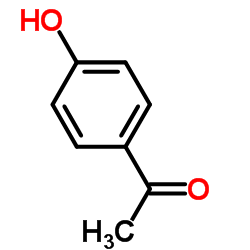 |
4-Hydroxyacetophenone
CAS:99-93-4 |
|
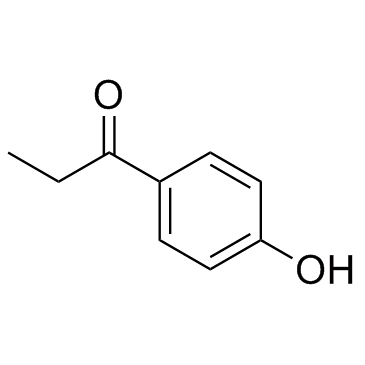 |
1-(4-Hydroxyphenyl)propan-1-one
CAS:70-70-2 |