1-(4-Hydroxyphenyl)propan-1-one
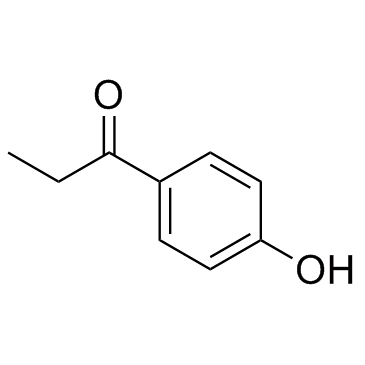
1-(4-Hydroxyphenyl)propan-1-one structure
|
Common Name | 1-(4-Hydroxyphenyl)propan-1-one | ||
|---|---|---|---|---|
| CAS Number | 70-70-2 | Molecular Weight | 150.174 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 152-154 °C26 mm Hg(lit.) | |
| Molecular Formula | C9H10O2 | Melting Point | 36-38 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | >230 °F | |
|
Convenient QSAR model for predicting the complexation of structurally diverse compounds with β-cyclodextrins
Bioorg. Med. Chem. 17 , 896-904, (2009) This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoretical molecular descriptors, calculated solely from the mole... |
|
|
Development of a phospholipidosis database and predictive quantitative structure-activity relationship (QSAR) models.
Toxicol. Mech. Methods 18 , 217-27, (2008) ABSTRACT Drug-induced phospholipidosis (PL) is a condition characterized by the accumulation of phospholipids and drug in lysosomes, and is found in a variety of tissue types. PL is frequently manifested in preclinical studies and may delay or prevent the dev... |
|
|
Inhibition of GABA shunt enzymes' activity by 4-hydroxybenzaldehyde derivatives.
Bioorg. Med. Chem. Lett. 16 , 592-5, (2006) 4-Hydroxybenzaldehyde (HBA) derivatives were examined as inhibitors for GABA transaminase (GABA-T) and succinic semialdehyde dehydrogenase (SSADH). Investigation of structure-activity relation revealed that a carbonyl group or an amino group as well as a hydr... |
|
|
Synthesis, biochemical evaluation and rationalisation of the inhibitory activity of a series of 4-hydroxyphenyl ketones as potential inhibitors of 17beta-hydroxysteroid dehydrogenase type 3 (17beta-HSD3).
Bioorg. Med. Chem. Lett. 16 , 4519-22, (2006) We report the preliminary results of the synthesis and biochemical evaluation of a number of 4-hydroxyphenyl ketones as inhibitors of the isozyme of the enzyme 17beta-hydroxysteroid dehydrogenase (17beta-HSD) responsible for the conversion of androstenedione ... |
|
|
[Changes in basic nitrogen in a group of potential new beta- adrenolytics--derivatives of p-hydroxyacetophenone and p-hydroxypropiofenone].
Cesk. Farm. 42(2) , 82-5, (1993) Within the relationship of the structure and effect of new beta-adrenolytic agents derivatived from p-hydroxyacetophenone and p-hydroxypropiophenone with a propoxymethyl group in the lipophilic part of the molecule and with a propanamine, a butanamine and a p... |
|
|
[Anti-calcium effects of 4-hydroxypropiophenone derivatives--potential beta-adrenoreceptor blockers].
Ceska. Slov. Farm. 43(5) , 226-8, (1994) The present paper carries out the pharmacological evaluation of 4-(2-hydroxy-3-isopropylaminopropoxy)-3-(alkoxymethyl) propiophenones with an ethoxy, propoxy and butoxy-group, whose structures are typical of the blockers of beta-adrenergic receptors. In the a... |
|
|
[Derivatives of para-hydroxyacetophenone and para-hydroxypropiophenone as a potential new beta-adrenolytic agent].
Cesk. Farm. 39(9) , 403-8, (1990) Within the framework of the study of the structure-effect relationship, a series of novel potential beta-adrenolytic agents derived from p-hydroxyacetophenone and p-hydroxypropiophenone with an alyloxymethyl and a cycloalkyloxymethyl group in the lipophilic p... |
|
|
Conversion of 4-hydroxyacetophenone into 4-phenyl acetate by a flavin adenine dinucleotide-containing Baeyer-Villiger-type monooxygenase.
J. Bacteriol. 182(23) , 6565-9, (2000) An arylketone monooxygenase was purified from Pseudomonas putida JD1 by ion exchange and affinity chromatography. It had the characteristics of a Baeyer-Villiger-type monooxygenase and converted its substrate, 4-hydroxyacetophenone, into 4-hydroxyphenyl aceta... |
|
|
Inhibitory effects of ethacrynic acid analogues lacking the α,β-unsaturated carbonyl unit and para-acylated phenols on human cancer cells.
Bioorg. Med. Chem. Lett. 21 , 912-5, (2011) A series of ethacrynic acid analogues, lacking the α,β-unsaturated carbonyl unit, was synthesized and subsequently evaluated for their ability to inhibit the migration of human breast cancer cells, Hs578Ts(i)8 as well as of human prostate cancer cells, C4-2B.... |
|
|
Chemiexcitation in the peroxidative metabolism of diethylstilbestrol. Metabolic products.
Photochem. Photobiol. 55(2) , 267-77, (1992) In the presence of the surfactant hexadecyltrimethyl ammonium bromide (CTAB) a cascade of electronically excited states accompanies the successive steps in the peroxidative metabolization of the strong estrogenic and tumourogenic diethylstilbestrol. Reversing... |