Inhibitory effects of ethacrynic acid analogues lacking the α,β-unsaturated carbonyl unit and para-acylated phenols on human cancer cells.
Zack E Bryant, Romy F J Janser, Medina Jabarkhail, Melissa S Candelaria-Lyons, Brittni B Romero, Severine Van slambrouck, Wim F A Steelant, Ingo Janser
Index: Bioorg. Med. Chem. Lett. 21 , 912-5, (2011)
Full Text: HTML
Abstract
A series of ethacrynic acid analogues, lacking the α,β-unsaturated carbonyl unit, was synthesized and subsequently evaluated for their ability to inhibit the migration of human breast cancer cells, Hs578Ts(i)8 as well as of human prostate cancer cells, C4-2B. These cell lines provide a good model system to study migration and invasion, since they represent metastatic cancer. Our studies show that ethacrynic acid analogues with methyl substituents at the aromatic ring demonstrate no inhibitory effect on the migration of both cancer cell lines, whereas a precursor in the synthesis of these ethacrynic acid analogues (II-1, a para-acylated m-cresol) is an excellent inhibitor of the migration of both cancer cell lines.Copyright © 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
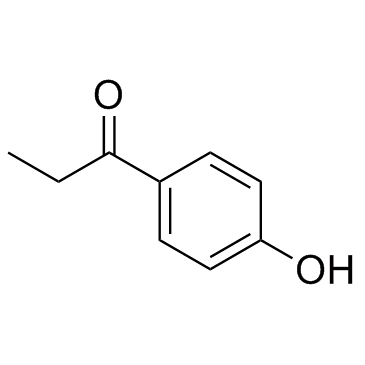 |
1-(4-Hydroxyphenyl)propan-1-one
CAS:70-70-2 |
C9H10O2 |
|
Convenient QSAR model for predicting the complexation of str...
2009-01-01 [Bioorg. Med. Chem. 17 , 896-904, (2009)] |
|
Development of a phospholipidosis database and predictive qu...
2008-01-01 [Toxicol. Mech. Methods 18 , 217-27, (2008)] |
|
Inhibition of GABA shunt enzymes' activity by 4-hydroxybenza...
2006-02-01 [Bioorg. Med. Chem. Lett. 16 , 592-5, (2006)] |
|
Synthesis, biochemical evaluation and rationalisation of the...
2006-09-01 [Bioorg. Med. Chem. Lett. 16 , 4519-22, (2006)] |
|
[Changes in basic nitrogen in a group of potential new beta-...
1993-04-01 [Cesk. Farm. 42(2) , 82-5, (1993)] |