2,5-Biphenyldiol
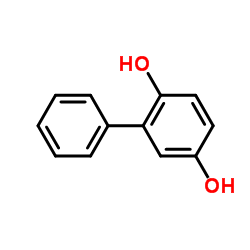
2,5-Biphenyldiol structure
|
Common Name | 2,5-Biphenyldiol | ||
|---|---|---|---|---|
| CAS Number | 1079-21-6 | Molecular Weight | 186.207 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 383.0±30.0 °C at 760 mmHg | |
| Molecular Formula | C12H10O2 | Melting Point | 98-100 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 192.3±19.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Cytotoxic effects of phenyl-hydroquinone and some hydroquinones on isolated rat hepatocytes.
Biochem. Pharmacol. 44(6) , 1059-65, (1992) The cytotoxic effects of phenyl-hydroquinone (PHQ) and some other hydroquinones on freshly isolated rat hepatocytes were investigated. Addition of PHQ (0.5 or 0.75 mM) to the hepatocytes elicited dose-dependent cell death accompanied by losses of intracellula... |
|
|
Tuning surface hydrophilicity/hydrophobicity of hydrocarbon proton exchange membranes (PEMs).
J. Colloid. Interface Sci. 466 , 168-77, (2016) The effect of annealing on the surface hydrophilicity of various representative classes of hydrocarbon-based proton exchange membranes (PEMs) is investigated. In all cases, a more hydrophilic membrane surface develops after annealing at elevated temperatures.... |
|
|
Metabolism of phenylhydroquinone by prostaglandin (H) synthase: possible implications in o-phenylphenol carcinogenesis.
Carcinogenesis 12(1) , 145-9, (1991) o-Phenylphenol (OPP) and its sodium salt sodium ortho-phenylphenate (NaOPP) are broad spectrum fungicides and antibacterial agents. Both are urinary bladder and renal carcinogens in the Fischer 344 rat. OPP is converted by mixed-function oxidases in the liver... |
|
|
The inhibition of phenylhydroquinone-induced oxidative DNA cleavage by constituents of Moutan Cortex and Paeoniae Radix.
Biol. Pharm. Bull. 23(2) , 199-203, (2000) Moutan Cortex (root cortex of Paeonia suffruticosa ANDREWS) and Paeoniae Radix (root of Paeonia lactiflora PALLAS) are crude drugs used in many traditional prescriptions and have constituents in common. We studied the effects of extracts of these crude drugs ... |
|
|
Metabolites of the biocide o-phenylphenol generate oxidative DNA lesions in V 79 cells.
Arch. Toxicol. 73(10-11) , 607-10, (2000) Incubation of the o-phenylphenol (OPP) metabolites, o-phenylhydroquinone (PHQ) and o-phenylbenzoquinone (PBQ) with V 79 Chinese hamster cells led to a significant enhancement of the amount of 8-hydroxy-2'-deoxyguanosine (8-OH-dG) in nuclear DNA. With OPP no d... |
|
|
DNA adduct formation by o-phenylphenol metabolite in vivo and in vitro.
Carcinogenesis 13(8) , 1469-73, (1992) [U-14C]o-Phenylphenol (OPP) was found to bind covalently to calf thymus DNA during a 60 min incubation in the presence of microsomes, but not in their absence, indicating that metabolic conversion of the parent compound, OPP, to an activated form is essential... |
|
|
Peroxidative activation of o-phenylhydroquinone leads to the formation of DNA adducts in HL-60 cells.
Carcinogenesis 13(10) , 1937-9, (1992) Using 32P-postlabeling we studied DNA adduct formation in HL-60 cells treated with the o-phenylphenol metabolites o-phenylhydroquinone (o-PHQ) and o-phenylbenzoquinone (o-PBQ). Treatment with 25-500 microM o-PHQ for 8 h produced one principal and three minor ... |
|
|
Induction of 8-hydroxy-2'-deoxyguanosine in CHO-K1 cells exposed to phenyl-hydroquinone, a metabolite of ortho-phenylphenol.
Cancer Lett. 101(2) , 227-32, (1996) The induction of 8-hydroxy-2'-deoxyguanosine (8-OHdG), an index of oxidative DNA modification, was investigated in CHO-K1 cells exposed to phenyl-hydroquinone (PHQ), a major metabolite of ortho-phenylphenol (OPP), an antimicrobial. Addition of PHQ at a concen... |
|
|
Analysis of genotoxicity and the carcinogenic mode of action for ortho-phenylphenol.
Environ. Mol. Mutagen. 45(5) , 460-81, (2005) Ortho-phenylphenol (OPP) and its sodium salt (SOPP) are commercial products that have wide human exposure and have been shown in several studies to be rodent carcinogens. Genetic toxicology data were assessed in an attempt to understand the carcinogenic mode ... |
|
|
Formation of 8-hydroxydeoxyguanosine in calf thymus DNA treated in vitro with phenylhydroquinone, the major metabolite of O-phenylphenol.
Carcinogenesis 16(4) , 837-40, (1995) The generation of 8-hydroxydeoxyguanosine (8OHdG) in calf thymus DNA treated with O-phenylphenol (OPP) or its major metabolites, phenylhydroquinone (PHQ) and phenylbenzoquinone (PBQ), was studied. The content of 8OHdG residues was increased in DNA treated wit... |