Aspergillus acid protease
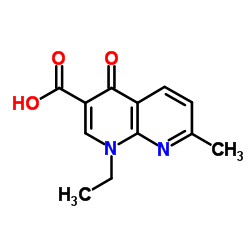
Aspergillus acid protease structure
|
Common Name | Aspergillus acid protease | ||
|---|---|---|---|---|
| CAS Number | 9025-49-4 | Molecular Weight | 232.235 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 413.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C12H12N2O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 203.6±28.7 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
|
[Modification of two tyrosine residues in aspergillopepsin A by p-nitrophenyldiazonium chloride].
Biokhimiia 46(2) , 369-75, (1981) p-Nitrophenyldiazonium chloride was found to modify the Tyr-75 and Tyr-189 residues in aspergillopepsin A. Incubation of the protein with a 45-fold molar excess of the reagent at pH 5,2 results in the attachment of 1,8 inhibitor residues, which leads to a 75%... |
|
|
Sequences from the aspergillopepsin PEP gene of Aspergillus fumigatus: evidence on their use in selective PCR identification of Aspergillus species in infected clinical samples.
FEMS Immunol. Med. Microbiol. 25(3) , 255-64, (1999) In immunodeficient patients, Aspergillus species emerge as circumstantial pathogens. Aspergillus fumigatus is a distant first among the pathogenic aspergilli, which cause deep-seated mycoses. Sequences of the pep gene of A. fumigatus as potential PCR primers,... |
|
|
The site of diazoacetyl inhibitor attachment to acid proteinase of Aspergillus awamori--an analog of penicillopepsin and pepsin.
Biochem. Biophys. Res. Commun. 49(4) , 1075-81, (1972)
|
|
|
Isolation and characterization of mutants of Aspergillus niger deficient in extracellular proteases.
Mol. Gen. Genet. 234(2) , 332-6, (1992) In the present study, the extracellular protease activity in a strain of the filamentous fungus Aspergillus niger was investigated and mutant strains deficient in the production of extracellular proteases were isolated. The major protease, which is responsibl... |
|
|
Molecular cloning of a cDNA for proctase B from Aspergillus niger var. macrosporus and sequence comparison with other aspergillopepsins I.
Biosci. Biotechnol. Biochem. 59(5) , 954-5, (1995) A cDNA for proctase B from Aspergillus niger var. macrosporus was isolated and sequenced. The deduced amino acid sequence (394 residues) of the preproform of the enzyme was highly homologous (98% identify) with those of aspergillopepsins I from A. awamori and... |
|
|
Purification of an acid proteinase from Aspergillus saitoi and determination of peptide bond specificity.
Biochim. Biophys. Acta 485(2) , 406-16, (1977) The specificity and mode of action of an acid proteinase (EC 3.4.23.6) from Aspergillus saitoi were investigated with oxidized B-chain of insulin, angiotensin II and bradykinin. Further purification of acid proteinase was performed with N,O-dibenzyloxycarbony... |
|
|
Kinetic study on the interaction of Rhizopus chinensis aspartic protease with Streptomyces pepsin inhibitor (acetylpepstatin).
Arch. Biochem. Biophys. 263(2) , 311-4, (1988) The fluorescence of tryptophan residues of Rhizopus chinensis aspartic protease was quenched about 25% upon binding with an inhibitor, Streptomyces pepsin inhibitor (acetylpepstatin). The kinetics of binding between the enzyme and the inhibitor was studied by... |
|
|
Characterization of the S1 subsite specificity of aspergillopepsin I by site-directed mutagenesis.
J. Biochem. 120(5) , 974-81, (1996) The structural determinants of S1 substrate specificity of aspergillopepsin I (API; EC 3.4.23.18), an aspartic proteinase from Aspergillus saitoi, were investigated by site-directed mutagenesis. Aspartic proteinases generally favor hydrophobic amino acids at ... |
|
|
Expression of a synthetic copy of the bovine chymosin gene in Aspergillus awamori from constitutive and pH-regulated promoters and secretion using two different pre-pro sequences.
Biotechnol. Bioeng. 83(3) , 249-59, (2003) A copy of the bovine chymosin gene (chy) with a codon usage optimized for its expression in Aspergillus awamori was constructed starting from synthetic oligonucleotides. To study the ability of this filamentous fungus to secrete bovine prochymosin, two plasmi... |
|
|
Overexpression and lack of degradation of thaumatin in an aspergillopepsin A-defective mutant of Aspergillus awamori containing an insertion in the pepA gene.
Appl. Microbiol. Biotechnol. 54(6) , 772-7, (2000) A gene encoding the sweet-tasting protein thaumatin (tha) with optimized codon usage was expressed in Aspergillus awamori. Mutants of A. awamori with reduced proteolytic activity were isolated. One of these mutants, named lpr66, contained an insertion of abou... |