Kinetic study on the interaction of Rhizopus chinensis aspartic protease with Streptomyces pepsin inhibitor (acetylpepstatin).
H Nakatani, K Hiromi, K Kitagishi
Index: Arch. Biochem. Biophys. 263(2) , 311-4, (1988)
Full Text: HTML
Abstract
The fluorescence of tryptophan residues of Rhizopus chinensis aspartic protease was quenched about 25% upon binding with an inhibitor, Streptomyces pepsin inhibitor (acetylpepstatin). The kinetics of binding between the enzyme and the inhibitor was studied by the fluorescence stopped-flow method. The concentration dependence of apparent rate constants was consistent with a two-step mechanism involving a fast bimolecular association followed by a slow unimolecular process. The unimolecular process was interpreted to be a conversion from a transient intermediate to the final complex in which the inhibitor is tightly bound to the active site of the enzyme. Fluorescence quenching occurred essentially in the unimolecular process, which suggests microenvironmental transition around at least one tryptophan residue in the enzyme-substrate complex.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
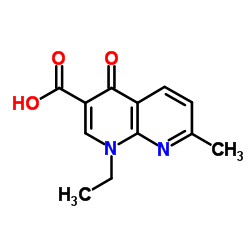 |
Aspergillus acid protease
CAS:9025-49-4 |
C12H12N2O3 |
|
[Modification of two tyrosine residues in aspergillopepsin A...
1981-02-01 [Biokhimiia 46(2) , 369-75, (1981)] |
|
Sequences from the aspergillopepsin PEP gene of Aspergillus ...
1999-08-15 [FEMS Immunol. Med. Microbiol. 25(3) , 255-64, (1999)] |
|
The site of diazoacetyl inhibitor attachment to acid protein...
1972-11-15 [Biochem. Biophys. Res. Commun. 49(4) , 1075-81, (1972)] |
|
Isolation and characterization of mutants of Aspergillus nig...
1992-08-01 [Mol. Gen. Genet. 234(2) , 332-6, (1992)] |
|
Molecular cloning of a cDNA for proctase B from Aspergillus ...
1995-05-01 [Biosci. Biotechnol. Biochem. 59(5) , 954-5, (1995)] |