4-Bromomethylbenzoic acid
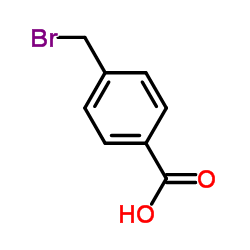
4-Bromomethylbenzoic acid structure
|
Common Name | 4-Bromomethylbenzoic acid | ||
|---|---|---|---|---|
| CAS Number | 6232-88-8 | Molecular Weight | 215.044 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 328.8±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H7BrO2 | Melting Point | 224-229 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 152.7±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
CO₂ controlled flocculation of microalgae using pH responsive cellulose nanocrystals.
Nanoscale 7 , 14413-21, (2015) Cellulose nanocrystals were grafted with imidazole functionalities up to DS 0.06 using a one-pot functionalization strategy. The resulting nanocrystals were shown to have a pH responsive surface charge which was found to be positive below pH 6 and negative ab... |
|
|
Highly charged cellulose-based nanocrystals as flocculants for harvesting Chlorella vulgaris.
Bioresour. Technol. 194 , 270-5, (2015) This study presents a novel flocculant for harvesting Chlorella vulgaris as model species for freshwater microalgae based on cellulose nanocrystals (CNCs), thus synthesized from a renewable and biodegradable resource. Cationic pyridinium groups were grafted o... |
|
|
5-Arylidene-2,4-thiazolidinediones as inhibitors of protein tyrosine phosphatases.
Bioorg. Med. Chem. 15(15) , 5137-49, (2007) 4-(5-Arylidene-2,4-dioxothiazolidin-3-yl)methylbenzoic acids (2) were synthesized and evaluated in vitro as inhibitors of PTP1B and LMW-PTP, two protein tyrosine phosphatases (PTPs) which act as negative regulators of the metabolic and mitotic signalling of i... |
|
|
Chemical modification of temoporfin--a second generation photosensitizer activated using upconverting nanoparticles for singlet oxygen generation.
Chem. Commun. (Camb.) 50(81) , 12150-3, (2014) LiYF4:Tm(3+)/Yb(3+) upconverting nanoparticles (UCNPs) were functionalized with the second generation photosensitizer 5,10,15,20-tetra(m-hydroxyphenyl)chlorin (m-THPC, Temoporfin, Foscan®). m-THPC was modified using 4-(bromomethyl)benzoic acid, which induced ... |
|
|
Synthesis and evaluation of the anti parasitic activity of aromatic nitro compounds.
Eur. J. Med. Chem. 46 , 5443-7, (2011) A series of nitroaromatic compounds was synthesized and evaluated as potential antileishmanial and trypanocidal agents. Five compounds exerted significant anti-leishmanial activity in vitro against promastigotes forms of Leishmania (L.) amazonensis, with IC(5... |