| Structure | Name/CAS No. | Articles |
|---|---|---|
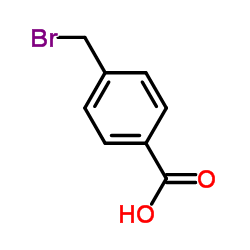 |
4-Bromomethylbenzoic acid
CAS:6232-88-8 |
Rosanna Maccari, Paolo Paoli, Rosaria Ottanà, Michela Jacomelli, Rosella Ciurleo, Giampaolo Manao, Theodora Steindl, Thierry Langer, Maria Gabriella Vigorita, Guido Camici
Index: Bioorg. Med. Chem. 15(15) , 5137-49, (2007)
Full Text: HTML
4-(5-Arylidene-2,4-dioxothiazolidin-3-yl)methylbenzoic acids (2) were synthesized and evaluated in vitro as inhibitors of PTP1B and LMW-PTP, two protein tyrosine phosphatases (PTPs) which act as negative regulators of the metabolic and mitotic signalling of insulin. The synthesis of compounds 2 represents an example of utilizing phosphotyrosine-mimetics to identify effective low molecular weight nonphosphorus inhibitors of PTPs. Several thiazolidinediones 2 exhibited PTP1B inhibitory activity in the low micromolar range with moderate selectivity for human PTP1B and IF1 isoform of human LMW-PTP compared with other related PTPs.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
4-Bromomethylbenzoic acid
CAS:6232-88-8 |
C8H7BrO2 |
|
CO₂ controlled flocculation of microalgae using pH responsiv...
2015-09-14 [Nanoscale 7 , 14413-21, (2015)] |
|
Highly charged cellulose-based nanocrystals as flocculants f...
2015-10-01 [Bioresour. Technol. 194 , 270-5, (2015)] |
|
Chemical modification of temoporfin--a second generation pho...
2014-10-18 [Chem. Commun. (Camb.) 50(81) , 12150-3, (2014)] |
|
Synthesis and evaluation of the anti parasitic activity of a...
2011-11-01 [Eur. J. Med. Chem. 46 , 5443-7, (2011)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved