ALLYL CHLORIDE
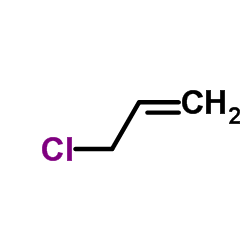
ALLYL CHLORIDE structure
|
Common Name | ALLYL CHLORIDE | ||
|---|---|---|---|---|
| CAS Number | 107-05-1 | Molecular Weight | 76.525 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 41.6±9.0 °C at 760 mmHg | |
| Molecular Formula | C3H5Cl | Melting Point | -136 °C | |
| MSDS | Chinese USA | Flash Point | -28.9±0.0 °C | |
| Symbol |




GHS02, GHS06, GHS08, GHS09 |
Signal Word | Danger | |
|
Halogenated derivatives QSAR model using spectral moments to predict haloacetic acids (HAA) mutagenicity.
Bioorg. Med. Chem. 16 , 5720-32, (2008) The risk of the presence of haloacetic acids in drinking water as chlorination by-products and the shortage of experimental mutagenicity data for most of them requires a research work. This paper describes a QSAR model to predict direct mutagenicity for these... |
|
|
Regio- and enantioselective o-allylation of phenol and alcohol catalyzed by a planar-chiral cyclopentadienyl ruthenium complex.
Angew. Chem. Int. Ed. Engl. 47(8) , 1454-7, (2008)
|
|
|
A serendipitous synthesis of (+)-gregatin B, second structure revisions of the aspertetronins, gregatins, and graminin A, structure revision of the penicilliols.
Org. Lett. 13(10) , 2730-3, (2011) A (DHQN)(2)AQN-promoted asymmetric dihydroxylation (92% ee) of the allyl chloride derived from enynol (E)-13 and an 8-step sequence provided access to the hydroxyethylated furanone (R)-21. Oxidation with MnO(2) furnished 50% furanone (+)-(R)-2a and 2.7% isome... |
|
|
Syntheses of trisila analogues of allyl chlorides and their transformations to chlorocyclotrisilanes, cyclotrisilanides, and a trisilaindane.
J. Am. Chem. Soc. 130(12) , 4114-21, (2008) The rearrangements of (chlorosilyl)disilenes R2(Cl)Si-(Tip)Si=SiTip2 (5a,b: Tip = 2,4,6-iPr3C6H2, a: R = Me, b: R = Ph) quantitatively yield the isomeric chlorocyclotrisilanes (6a,b). The disilene precursors 5a,b are, in turn, accessible from the reactions of... |
|
|
Enabling olefin metathesis on proteins: chemical methods for installation of S-allyl cysteine.
Chem. Commun. (Camb.) (25) , 3714-6, (2009) Multiple, complementary methods are reported for the chemical conversion of cysteine to S-allyl cysteine on protein surfaces, a useful transformation for the exploration of olefin metathesis on proteins. |
|
|
Malondialdehyde and catalase as the serum biomarkers of allyl chloride-induced toxic neuropathy.
Toxicology 227(1-2) , 36-44, (2006) Chronic exposure to allyl chloride (AC) is known to produce a central-peripheral distal axonopathy. To access the biomarker of exposure and elucidate the mechanism of neuropathy induced by AC, we performed a longitudinal observational study of malondialdehyde... |
|
|
Expression changes of apoptotic-related proteins in nerve tissues of rats treated with allyl chloride.
Toxicology 231(1) , 58-67, (2007) Allyl chloride (AC) is widely used in industries as raw material and has been reported to produce occupational peripheral neuropathies in man chronically exposure to it. Although many studies have been done addressing to it, the mechanisms still remain unclea... |
|
|
Nickel-catalyzed coupling of allyl chlorides and enynes.
Chem. Commun. (Camb.) (4) , 457-9, (2006) The Ni-catalyzed coupling of allyl chlorides and enynes has been developed; the cyclization of enynes was triggered by the addition of pi-allylnickel species to the alkyne part, followed by the incorporation of the alkene part. |
|
|
Modifications of neurofilament proteins by possible metabolites of allyl chloride in vitro.
Drug Chem. Toxicol. 18(4) , 315-31, (1995) Chronic exposure to allyl chloride (ALL) is known to produce a central-peripheral distal axonopathy. In relation to the mechanism(s), the present study was conducted to examine the abilities of ALL and its putative metabolites, i.e., epichlorohydrin, glycerol... |
|
|
Mechanism of allyl chloride-induced cytoskeletal injury to nerve cells.
Chin. Med. J. 111(6) , 556-9, (1998) To dissect the molecular mechanism of toxic neuropathy induced by allyl chloride (AC).Fluorescence molecular probe (Fura-2/AM), electron probe X-ray microprobe analysis (EPMA) and biochemical methods were used to determine the concentrations of cytosolic free... |