Organic Letters
2011-05-20
A serendipitous synthesis of (+)-gregatin B, second structure revisions of the aspertetronins, gregatins, and graminin A, structure revision of the penicilliols.
Heike Burghart-Stoll, Reinhard Brückner
Index: Org. Lett. 13(10) , 2730-3, (2011)
Full Text: HTML
Abstract
A (DHQN)(2)AQN-promoted asymmetric dihydroxylation (92% ee) of the allyl chloride derived from enynol (E)-13 and an 8-step sequence provided access to the hydroxyethylated furanone (R)-21. Oxidation with MnO(2) furnished 50% furanone (+)-(R)-2a and 2.7% isomeric furanone (+)-(R)-3a. (R)-2a possesses the accepted constitution of (+)-gregatin B but its spectra are different. Surprisingly, (+)-(R)-3a equals the natural product. Analogous structure reassignments are due for the gregatins A and C-E, the aspertetronins A-B, graminin A, and the penicilliols A and B.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
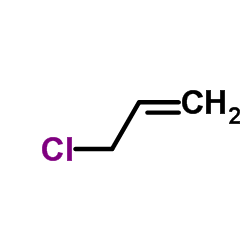 |
ALLYL CHLORIDE
CAS:107-05-1 |
C3H5Cl |
Related Articles:
More...
|
Halogenated derivatives QSAR model using spectral moments to...
2008-05-15 [Bioorg. Med. Chem. 16 , 5720-32, (2008)] |
|
Regio- and enantioselective o-allylation of phenol and alcoh...
2008-01-01 [Angew. Chem. Int. Ed. Engl. 47(8) , 1454-7, (2008)] |
|
Syntheses of trisila analogues of allyl chlorides and their ...
2008-03-26 [J. Am. Chem. Soc. 130(12) , 4114-21, (2008)] |
|
Enabling olefin metathesis on proteins: chemical methods for...
2009-07-07 [Chem. Commun. (Camb.) (25) , 3714-6, (2009)] |
|
Malondialdehyde and catalase as the serum biomarkers of ally...
2006-10-03 [Toxicology 227(1-2) , 36-44, (2006)] |