利托那韦
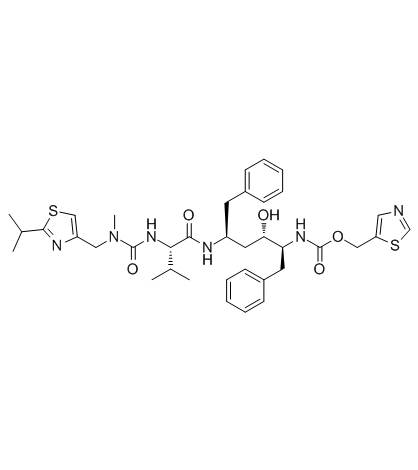
利托那韦结构式

|
常用名 | 利托那韦 | 英文名 | Ritonavir |
|---|---|---|---|---|
| CAS号 | 155213-67-5 | 分子量 | 720.944 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 947.0±65.0 °C at 760 mmHg | |
| 分子式 | C37H48N6O5S2 | 熔点 | 120-122°C | |
| MSDS | 中文版 美版 | 闪点 | 526.6±34.3 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
In vitro and in vivo release characteristics of Tacrolimus (FK506) from an episcleral drug-delivery implant.
J. Ocul. Pharmacol. Ther. 30(8) , 670-80, (2014) To investigate the in vitro and in vivo release characteristics of Tacrolimus (FK506) from an episcleral drug-delivery implant.For in vitro experiments, Tacrolimus-loaded implants (0.5 mL; at concentrations of 0.25, 0.5, and 1.0 mg/mL) were immersed in a bala... |
|
|
Cell-free microfluidic determination of P-glycoprotein interactions with substrates and inhibitors.
Pharm. Res. 31(12) , 3415-25, (2014) The membrane protein P-glycoprotein (P-gp) plays key roles in the oral bioavailability of drugs, their blood brain barrier passage as well as in multidrug resistance. For new drug candidates it is mandatory to study their interaction with P-gp, according to F... |
|
|
Molecular cloning and functional characterization of a rainbow trout liver Oatp.
Toxicol. Appl. Pharmacol. 280(3) , 534-42, (2014) Cyanobacterial blooms have an impact on the aquatic ecosystem due to the production of toxins (e.g. microcystins, MCs), which constrain fish health or even cause fish death. However the toxicokinetics of the most abundant toxin, microcystin-LR (MC-LR), are no... |
|
|
Effect of ultra-high pressure homogenization on the interaction between bovine casein micelles and ritonavir.
Pharm. Res. 32(3) , 1055-71, (2015) The aim of this work was to develop a milk-based powder formulation appropriate for pediatric delivery of ritonavir (RIT).Ultra-high pressure homogenization (UHPH) at 0.1, 300 and 500 MPa was used to process a dispersion of pasteurized skim milk (SM) and rito... |
|
|
Unbound ritonavir concentrations in rat and human hepatocytes.
J. Pharm. Sci. 104 , 2378-87, (2015) Knowledge regarding intracellular drug exposure is crucial to gain mechanistic understanding of hepatic disposition. This study aims to develop an approach to determine unbound intracellular concentrations (Cu,cell ) of ritonavir. Ritonavir was selected as a ... |
|
|
Human hepatocyte assessment of imatinib drug-drug interactions - complexities in clinical translation.
Br. J. Clin. Pharmacol. 80 , 1097-108, (2015) Inducers and inhibitors of CYP3A, such as ritonavir and efavirenz, may be used as part of the highly active antiretroviral therapy (HAART) to treat HIV patients. HIV patients with chronic myeloid leukemia or gastrointestinal stromal tumour may need imatinib, ... |
|
|
Potential interactions between HIV drugs, ritonavir and efavirenz and anticancer drug, nilotinib--a study in primary cultures of human hepatocytes that is applicable to HIV patients with cancer.
J. Clin. Pharmacol. 54(11) , 1272-9, (2014) Nilotinib is used to treat chronic myeloid leukemia (CML), and is metabolized by CYP3A. With a black-box warning for QT prolongation, which is exposure dependent, controlling for drug interactions is clinically relevant. Treatments of HIV patients with CML ar... |
|
|
Temporal endogenous gene expression profiles in response to lipid-mediated transfection.
J. Gene Med. 17(1-2) , 14-32, (2015) Design of efficient nonviral gene delivery systems is limited as a result of the rudimentary understanding of the specific molecules and processes that facilitate DNA transfer.Lipoplexes formed with Lipofectamine 2000 (LF2000) and plasmid-encoding green fluor... |
|
|
Decoding the anti-Trypanosoma cruzi action of HIV peptidase inhibitors using epimastigotes as a model.
PLoS ONE 9(12) , e113957, (2014) Aspartic peptidase inhibitors have shown antimicrobial action against distinct microorganisms. Due to an increase in the occurrence of Chagas' disease/AIDS co-infection, we decided to explore the effects of HIV aspartic peptidase inhibitors (HIV-PIs) on Trypa... |
|
|
Amorphous Solid Dispersions: Utilization and Challenges in Drug Discovery and Development.
J. Pharm. Sci. 104 , 3237-58, (2015) Amorphous solid dispersion (ASD) can accelerate a project by improving dissolution rate and solubility, offering dose escalation flexibility and excipient acceptance for toxicology studies, as well as providing adequate preclinical and clinical exposure. The ... |