| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
盐酸
CAS:7647-01-0 |
|
 |
十二烷基硫酸钠
CAS:151-21-3 |
|
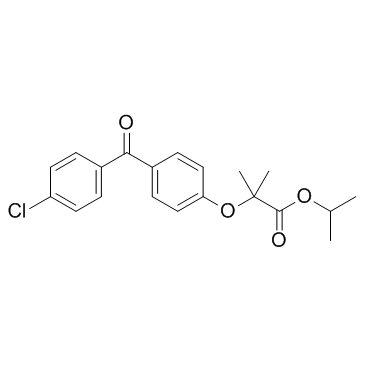 |
非诺贝特
CAS:49562-28-9 |
|
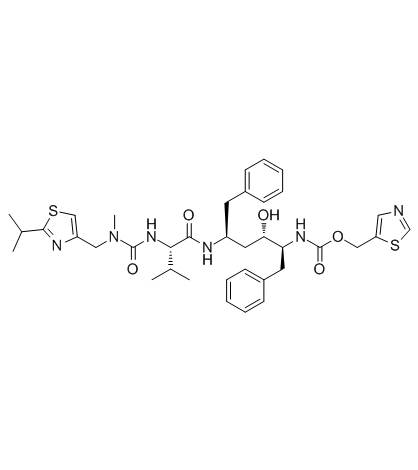 |
利托那韦
CAS:155213-67-5 |
|
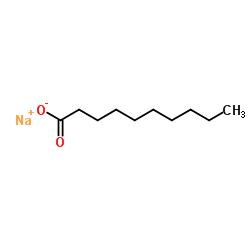 |
癸酸钠
CAS:1002-62-6 |
|
 |
灰黄霉素
CAS:126-07-8 |
|
 |
布洛芬
CAS:15687-27-1 |