布洛芬

布洛芬结构式

|
常用名 | 布洛芬 | 英文名 | Ibuprofen |
|---|---|---|---|---|
| CAS号 | 15687-27-1 | 分子量 | 206.281 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 319.6±11.0 °C at 760 mmHg | |
| 分子式 | C13H18O2 | 熔点 | 77-78 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 216.7±14.4 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
布洛芬用途Ibuprofen 是 COX-1 和 COX-2 的抑制剂,IC50 值分别为 13 μM 和 370 μM,具有抗炎的活性。 |
||||
布洛芬作用1、缓解类风湿关节炎、骨关节炎、脊柱关节病、痛风性关节炎、风湿性关节炎等各种慢性关节炎的急性发作期或持续性 的关节肿痛症状,无病因治疗及控制病程的作用。 2、治疗非关节性的各种软组织风湿性疼痛,如肩痛、腱鞘炎、滑囊炎、肌痛及运动后损伤性疼痛等。
3、急性的轻、中度疼痛如:手术后、创伤后、劳损后、原发性痛经、牙痛、头痛等。
4、对成人和儿童的发热有解热作用。 更多
|
| 中文名 | 布洛芬 |
|---|---|
| 英文名 | ibuprofen |
| 中文别名 | 4-异丁基-Α-甲基苯乙酸 | 异丁苯丙酸 | 对异丁苯丙酸 | 芬必得 | 2-(4-异丁基苯基)丙酸 | 2-(-4-异丁基苯基)丙酸 | 异丁洛芬 | 拔怒风 | 2-甲基-4-(2-甲基丙基)苯乙酸 |
| 英文别名 | 更多 |
| 描述 | Ibuprofen 是 COX-1 和 COX-2 的抑制剂,IC50 值分别为 13 μM 和 370 μM,具有抗炎的活性。 |
|---|---|
| 相关类别 | |
| 靶点 |
COX-1:13 μM (IC50) COX-2:370 μM (IC50) |
| 体外研究 | 布洛芬抑制酶环氧合酶COX-1和COX-2,其将花生四烯酸转化为前列腺素H2(PGH2)。其作用类似于阿司匹林,吲哚美辛和完整细胞,破碎细胞和纯化酶制剂中的所有其他NSAID [1]。布洛芬抑制雄激素非依赖性前列腺肿瘤细胞PC-3和DU-145中NF-κB和IKKα的组成型活化。它使前列腺细胞对电离辐射敏感,并在雄激素敏感的前列腺肿瘤细胞系LNCaP中暴露于TNFα或电离辐射后阻断NF-κB的刺激激活。这两者都不能直接归因于IκB-α激酶的抑制,而是抑制IKKα的上游调节因子[2]。布洛芬通过降低癌细胞的存活发挥抗癌作用。布洛芬比阿司匹林和对乙酰氨基酚更有效,并且与(R)-氟比洛芬和吲哚美辛在膀胱和其他器官细胞系中诱导p75NTR蛋白表达相当[3]。 |
| 体内研究 | 布洛芬与环氧合酶的血红素基团反应以防止花生四烯酸转化。事先在体内接触布洛芬可以完全保护环氧合酶免受血小板中阿司匹林的不可逆作用[4]。布洛芬治疗可有效减轻由高重复和高强度(HRHF)任务诱导的成年雌性Sprague-Dawley大鼠模型中的关节炎症和早期关节软骨退变。通过阻断血清C1和2C(胶原蛋白I和II降解的生物标志物)以及胶原蛋白降解与合成(C1,2C/CPII,后者是拼贴II型合成的生物标志物)诱导的比例来增加剂量。由HRHF [5]。 |
| 细胞实验 | 使用3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑(MTT)测定法估计用NSAID处理(48小时)后每个孔中的细胞数。将MTT标记试剂(终浓度,0.5 mg / mL)加入每种NSAID处理的T24细胞,单独使用ponasterone A的细胞,转染ΔDDp75NTR的细胞加ponasterone A,以及转染ΔICDp75NTR的细胞加ponasterone A(2×在96孔培养板(最终体积,100μL培养基/孔)中培养103个细胞/孔,并在37℃,10%CO 2的潮湿气氛中培养4小时。随后,将细胞与每孔100μL溶解溶液一起温育过夜,并使用微量滴定板读数器在570nm处定量样品。 |
| 动物实验 | 在任务执行的第4周结束时,上述动物的亚组每天在饮用水中施用布洛芬(45mg / kg体重):NC + IBU(n = 10),TR + IBU(n = 11)和HRHF + IBU(n = 15)。 HRHF + IBU动物在12周任务期的剩余时间内(即,布洛芬治疗的8周疗程)继续用布洛芬治疗进行HRHF任务方案。所用剂量低于大鼠胃肠道毒性的最大限度,但已被证明可有效减少慢性炎症。通过每天测量悬浮溶液的初始体积和最终体积之间的差异来跟踪每只动物消耗的含药水量/天。基于这些评估,所有组中平均每周布洛芬剂量相似(48.8±6.3mg / kg体重),布洛芬剂量施用或布洛芬血清水平在治疗组之间没有显着差异。 |
| 参考文献 |
| 密度 | 1.0±0.1 g/cm3 |
|---|---|
| 沸点 | 319.6±11.0 °C at 760 mmHg |
| 熔点 | 77-78 °C(lit.) |
| 分子式 | C13H18O2 |
| 分子量 | 206.281 |
| 闪点 | 216.7±14.4 °C |
| 精确质量 | 206.130676 |
| PSA | 37.30000 |
| LogP | 3.72 |
| 外观性状 | 无色,结晶固体 |
| 蒸汽压 | 0.0±0.7 mmHg at 25°C |
| 折射率 | 1.519 |
| 储存条件 | 常温密闭避光,通风干燥 |
| 稳定性 | 常温常压下稳定 避免的物料: 氧化物 |
| 水溶解性 | insoluble |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:1 3.氢键受体数量:2 4.可旋转化学键数量:4 5.互变异构体数量:无 6.拓扑分子极性表面积37.3 7.重原子数量:15 8.表面电荷:0 9.复杂度:203 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:1 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.性状:白色结晶性粉末。有异臭,无味。 2.密度(g/mL,25/4℃):未确定 3.相对蒸汽密度(g/mL,空气=1):未确定 4.熔点(ºC):77-78 5.沸点(ºC,常压):未确定 6.沸点(ºC,5.2kPa):未确定 7.折射率:未确定 8.闪点(ºC):未确定 9.比旋光度(º):未确定 10.自燃点或引燃温度(ºC):未确定 11.蒸气压(kPa,25ºC):未确定 12.饱和蒸气压(kPa,60ºC):未确定 13.燃烧热(KJ/mol):未确定 14.临界温度(ºC):未确定 15.临界压力(KPa):未确定 16.油水(辛醇/水)分配系数的对数值:未确定 17.爆炸上限(%,V/V):未确定 18.爆炸下限(%,V/V):未确定 19.溶解性:不溶于水 |
|
2-(4-异丁苯基)丙酸
修改号码:5
模块1. 化学品 产品名称: 2-(4-Isobutylphenyl)propionic Acid 修改号码: 5 模块2. 危险性概述 GHS分类 物理性危害未分类
健康危害 急性毒性(经口) 第4级 皮肤腐蚀/刺激 第2级 严重损伤/刺激眼睛 2A类 环境危害未分类 GHS标签元素 图标或危害标志 信号词警告 危险描述吞咽有害。 造成皮肤刺激 造成严重眼刺激 防范说明 [预防]使用本产品时切勿吃东西,喝水或吸烟。 处理后要彻底清洗双手。 穿戴防护手套/护目镜/防护面具。 [急救措施] 食入:若感不适,呼叫解毒中心/医生。漱口。 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。 眼睛接触:求医/就诊 皮肤接触:用大量肥皂和水轻轻洗。 若皮肤刺激:求医/就诊。 脱掉被污染的衣物,清洗后方可重新使用。 [废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。 2-(4-异丁苯基)丙酸 修改号码:5 模块3. 成分/组成信息 单一物质/混和物单一物质 化学名(中文名): 2-(4-异丁苯基)丙酸 百分比: >98.0%(GC)(T) CAS编码: 15687-27-1 俗名: Ibuprofen , 4-Isobutyl-α-methylphenylacetic Acid 分子式: C13H18O2 模块4. 急救措施 吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。 皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。 若皮肤刺激或发生皮疹:求医/就诊。 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。 如果眼睛刺激:求医/就诊。 食入: 若感不适,呼叫解毒中心/医生。漱口。 危害迹象: 发烧, 肝炎, 月经周期异常, 复视, 过敏, 肿胀, 皮炎 紧急救助者的防护:救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。 模块5. 消防措施 合适的灭火剂:干粉,泡沫,雾状水,二氧化碳 特定方法:从上风处灭火,根据周围环境选择合适的灭火方法。 非相关人员应该撤离至安全地方。 周围一旦着火:如果安全,移去可移动容器。 消防员的特殊防护用具:灭火时,一定要穿戴个人防护用品。 模块6. 泄漏应急处理 个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。 紧急措施:泄露区应该用安全带等圈起来,控制非相关人员进入。 环保措施:防止进入下水道。 控制和清洗的方法和材料:清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的 法律法规处置。 模块7. 操作处置与储存 处理 技术措施:在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手 和脸。 注意事项:如果粉尘或浮质产生,使用局部排气。 操作处置注意事项:避免接触皮肤、眼睛和衣物。 贮存 储存条件:保持容器密闭。存放于凉爽、阴暗处。 远离不相容的材料比如氧化剂存放。 包装材料:依据法律。 模块8. 接触控制和个体防护 工程控制:尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗 眼器。 个人防护用品 呼吸系统防护:防尘面具。依据当地和政府法规。 手部防护:防护手套。 眼睛防护:安全防护镜。如果情况需要,佩戴面具。 2-(4-异丁苯基)丙酸 修改号码:5 模块8. 接触控制和个体防护 皮肤和身体防护:防护服。如果情况需要,穿戴防护靴。 模块9. 理化特性 外形(20°C):固体 外观: 晶体-粉末 颜色:白色类白色 气味:特殊味 pH:无数据资料 熔点: 76°C 沸点/沸程 157 °C/0.5kPa 闪点:无资料 爆炸特性 爆炸下限:无资料 爆炸上限:无资料 蒸气压: 6.32x10-3Pa/25°C 密度:无资料 溶解度: [水] 不溶于(21mg/L, 25°C) [其他溶剂] 易溶于:酒精 溶于:许多有机溶剂 log水分配系数 = 3.97 模块10. 稳定性和反应性 化学稳定性:一般情况下稳定。 危险反应的可能性:未报道特殊反应性。 须避免接触的物质氧化剂 危险的分解产物: 一氧化碳, 二氧化碳 模块11. 毒理学信息 急性毒性: orl-chd LDLo:469 mg/kg orl-man LDLo:171 mg/kg orl-rat LD50:636 mg/kg orl-wmn TDLo:8 mg/kg 对皮肤腐蚀或刺激: skn-hmn 2%/2D 对眼睛严重损害或刺激:无资料 生殖细胞变异原性: cyt-mus-orl 1470 mg/kg/7D sce-mus-ipr 50 mg/kg sce-mus-orl 270 mg/kg 致癌性: IARC =无资料 NTP =无资料 生殖毒性: rec-rat TDLo:270 mg/kg(17-21D preg) orl-rat TDLo:9750 mg/kg(65D male) orl-wmn TDLo:8 mg/kg(1D pre) orl-mus TDLo:1260 mg/kg(7-13D preg) RTECS 号码: MU6640000 模块12. 生态学信息 生态毒性: 2-(4-异丁苯基)丙酸 修改号码:5 模块12. 生态学信息 鱼类:无资料 甲壳类:无资料 藻类:无资料 残留性 / 降解性:无资料 潜在生物累积 (BCF): 3 土壤中移动性 log水分配系数: 3.97 1.5 x 10-2 土壤吸收系数 (Koc): 亨利定律 3400 constant(PaM3/mol): 模块13. 废弃处置 如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中 焚烧。废弃处置时请遵守国家、地区和当地的所有法规。 模块14. 运输信息 联合国分类:与联合国分类标准不一致 UN编号:未列明 模块15. 法规信息 《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、 生产、储存、运输、装卸等方面均作了相应的规定。 模块16 - 其他信息 N/A |
| 符号 |

GHS07 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H302 |
| 警示性声明 | P301 + P312 + P330 |
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
| 危害码 (欧洲) | Xn:Harmful |
| 风险声明 (欧洲) | R22;R51/53;R63 |
| 安全声明 (欧洲) | S36-S61-S36/37 |
| 危险品运输编码 | 2811 |
| WGK德国 | 3 |
| RTECS号 | MU6640000 |
| 包装等级 | III |
| 危险类别 | 6.1(b) |
| 海关编码 | 2924299090 |
| 布洛芬上游产品 10 | |
|---|---|
| 布洛芬下游产品 10 | |
异丁基苯乙酮经缩合、水解、消除、氧化、中和而得。
| 海关编码 | 2916392000 |
|---|---|
| 中文概述 | 2916392000 布洛芬。监管条件:无。增值税率:17.0%。退税率:9.0%。最低关税:6.5%。普通关税:30.0% |
| 申报要素 | 品名, 成分含量, 用途, 丙烯酸、丙烯酸盐或酯应报明包装 |
| Summary | 2916392000 2-(4-isobutylphenyl)propanoic acid。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。Lowest tariff:6.5%。General tariff:30.0% |
|
Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU.
J. Chromatogr. A. 1418 , 140-9, (2015) The fate and removal of organic micropollutants in the environment is a demanding issue evidenced by the recent European policy. This work presents an analytical method for the trace quantification of... |
|
|
Validation of cyclooxygenase-2 as a direct anti-inflammatory target of 4-O-methylhonokiol in zymosan-induced animal models.
Arch. Pharm. Res. 38 , 813-25, (2015) 4-O-methylhonokiol (MH) is known to inhibit inflammation by partially understood mechanisms. Here, the anti-inflammatory mechanisms of MH were examined using enzymatic, cellular, and animal assays. In... |
|
|
Probiotic properties of lactic acid bacteria isolated from water-buffalo mozzarella cheese.
Probiotics Antimicrob Proteins 6 , 141-56, (2014) This study evaluated the probiotic properties (stability at different pH values and bile salt concentration, auto-aggregation and co-aggregation, survival in the presence of antibiotics and commercial... |
| Inoven |
| Lebrufen |
| (RS)-ibuprofen |
| IP-82 |
| Dolgit |
| Ibumetin |
| Femadon |
| 2-(4-Isobutylphenyl)propionic acid |
| QVY1&R D1Y1&1 |
| Para-Isobutylhydratropic Acid |
| rufen |
| EINECS 239-784-6 |
| Dolgin |
| rufin |
| Ibuprofen |
| Benzeneacetic acid, α-methyl-4-(2-methylpropyl)- |
| (±)-ibuprofen |
| Novogent N |
| Ibutid |
| fenbid |
| 2-(4-Isobutylphenyl)propanoic acid |
| MOTRIN |
| Andran |
| Bluton |
| Dolgirid |
| 4-Isobutyl-α-methylphenylacetic Acid |
| Amibufen |
| MFCD00010393 |
| Nurofen |
| Racemic ibuprofen |
| Advil |
| Brufen |
| Dolo-Dolgit |
| Seclodin |
| UNII:WK2XYI10QM |
| IbU |
| Panafen |
| Adran |

 CAS号41283-72-1
CAS号41283-72-1 CAS号201230-82-2
CAS号201230-82-2 CAS号63444-56-4
CAS号63444-56-4 CAS号40150-92-3
CAS号40150-92-3 CAS号62049-65-4
CAS号62049-65-4 CAS号51407-46-6
CAS号51407-46-6 CAS号108-20-3
CAS号108-20-3 CAS号60057-62-7
CAS号60057-62-7 CAS号36039-36-8
CAS号36039-36-8 CAS号58609-73-7
CAS号58609-73-7![2-[2-[4-(2-methylpropyl)phenyl]propanoylamino]acetic acid结构式](https://image.chemsrc.com/caspic/034/110467-58-8.png) CAS号110467-58-8
CAS号110467-58-8 CAS号31121-93-4
CAS号31121-93-4 CAS号40150-98-9
CAS号40150-98-9 CAS号38861-78-8
CAS号38861-78-8 CAS号43153-07-7
CAS号43153-07-7 CAS号100319-40-2
CAS号100319-40-2 CAS号65813-55-0
CAS号65813-55-0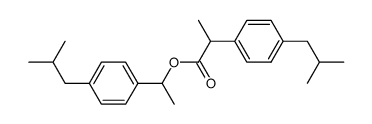 CAS号69474-20-0
CAS号69474-20-0
