Ibuprofen

Ibuprofen structure
|
Common Name | Ibuprofen | ||
|---|---|---|---|---|
| CAS Number | 15687-27-1 | Molecular Weight | 206.281 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 319.6±11.0 °C at 760 mmHg | |
| Molecular Formula | C13H18O2 | Melting Point | 77-78 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 216.7±14.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of IbuprofenIbuprofen is an anti-inflammatory inhibitor targeting COX-1 and COX-2 with IC50 of 13 μM and 370 μM, respectively. |
| Name | ibuprofen |
|---|---|
| Synonym | More Synonyms |
| Description | Ibuprofen is an anti-inflammatory inhibitor targeting COX-1 and COX-2 with IC50 of 13 μM and 370 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
COX-1:13 μM (IC50) COX-2:370 μM (IC50) |
| In Vitro | Ibuprofen inhibits the enzyme cyclooxygenase COX-1 and COX-2, which convert arachidonic acid to prostaglandin H2 (PGH2). Its action is similar to aspirin, indomethacin and all other NSAIDs in intact cells, broken cells, and purified enzyme preparations[1]. Ibuprofen inhibits the constitutive activation of NF-κB and IKKα in the androgen-independent prostate tumor cells PC-3 and DU-145. It sensitizes prostate cells to ionizing radiation and blocks stimulated activation of NF-κB following exposure to TNFα or ionizing radiation in the androgen-sensitive prostate tumor cell line LNCaP. Both of these cannot be attributed directly to inhibition of IκB-α kinase but to inhibition of an upstream regulator of IKKα[2]. Ibuprofen exerts an anticancer effect by reducing survival of cancer cells. Ibuprofen is more efficacious than aspirin and acetaminophen, and comparable with (R)-flurbiprofen and indomethacin in induction of p75NTR protein expression in cell lines from bladder and other organs[3]. |
| In Vivo | Ibuprofen reacts with the heme group of cyclooxygenase to prevent arachidonic acid conversion. Prior exposure to Ibuprofen in vivo protects cyclooxygenase completely from the irreversible effects of aspirin in platelets[4]. Ibuprofen treatment is effective in attenuating joint inflammation and early articular cartilage degeneration in the adult female Sprague-Dawley rat model induced by high-repetition and high-force (HRHF) task. It dose this by blocking the increases in serum C1 and 2C (a biomarker of collagen I and II degradation) as well as the ratio of collagen degradation to synthesis (C1, 2C/CPII, the latter a biomarker of collage type II synthesis) induced by HRHF[5]. |
| Cell Assay | The number of cells in each well after treatment (48 hours) with NSAIDs is estimated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT labeling reagent (final concentration, 0.5 mg/mL) is added to each of the NSAID-treated T24 cells, ponasterone A alone-treated cells, ΔDDp75NTR-transfected cells plus ponasterone A, and ΔICDp75NTR-transfected cells plus ponasterone A (2×103 cells/well) in 96-well culture plates (final volume, 100 μL culture medium/well) and incubated for 4 hours at 37°C in a humidified atmosphere of 10% CO2. Subsequently, cells are incubated overnight with 100 μL of solubilization solution per well, and the samples are quantified at 570 nm using a microtiter plate reader. |
| Animal Admin | At the end of the 4th week of task performance, subcohorts of the above animals are administered ibuprofen in drinking water daily (45 mg/kg body weight): NC+IBU (n=10), TR + IBU (n=11) and HRHF + IBU (n=15). HRHF+IBU animals continue to perform the HRHF task regimen with ibuprofen treatment for the remainder of the 12-week task period (i.e., an 8-week course of ibuprofen treatment). The dose used is lower than the maximum limit for gastrointestinal toxicity in rats, yet has been shown to be effective in reducing chronic inflammation. The amount of medicated water consumed/day is tracked for each animal by measuring the difference between the initial and final volume of suspended solution daily. Based on these assessments, the average weekly ibuprofen dose is similar in all groups (48.8±6.3 mg/kg body weight), with no significant differences in ibuprofen dose administered or serum levels of ibuprofen between the treated groups. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 319.6±11.0 °C at 760 mmHg |
| Melting Point | 77-78 °C(lit.) |
| Molecular Formula | C13H18O2 |
| Molecular Weight | 206.281 |
| Flash Point | 216.7±14.4 °C |
| Exact Mass | 206.130676 |
| PSA | 37.30000 |
| LogP | 3.72 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.519 |
| Storage condition | -20?C Freezer |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R51/53;R63 |
| Safety Phrases | S36-S61-S36/37 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| RTECS | MU6640000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2924299090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2916392000 |
|---|---|
| Summary | 2916392000 2-(4-isobutylphenyl)propanoic acid。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。Lowest tariff:6.5%。General tariff:30.0% |
|
Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU.
J. Chromatogr. A. 1418 , 140-9, (2015) The fate and removal of organic micropollutants in the environment is a demanding issue evidenced by the recent European policy. This work presents an analytical method for the trace quantification of... |
|
|
Validation of cyclooxygenase-2 as a direct anti-inflammatory target of 4-O-methylhonokiol in zymosan-induced animal models.
Arch. Pharm. Res. 38 , 813-25, (2015) 4-O-methylhonokiol (MH) is known to inhibit inflammation by partially understood mechanisms. Here, the anti-inflammatory mechanisms of MH were examined using enzymatic, cellular, and animal assays. In... |
|
|
Probiotic properties of lactic acid bacteria isolated from water-buffalo mozzarella cheese.
Probiotics Antimicrob Proteins 6 , 141-56, (2014) This study evaluated the probiotic properties (stability at different pH values and bile salt concentration, auto-aggregation and co-aggregation, survival in the presence of antibiotics and commercial... |
| Inoven |
| Lebrufen |
| (RS)-ibuprofen |
| IP-82 |
| Dolgit |
| Ibumetin |
| Femadon |
| 2-(4-Isobutylphenyl)propionic acid |
| QVY1&R D1Y1&1 |
| Para-Isobutylhydratropic Acid |
| rufen |
| EINECS 239-784-6 |
| Dolgin |
| rufin |
| Ibuprofen |
| Benzeneacetic acid, α-methyl-4-(2-methylpropyl)- |
| (±)-ibuprofen |
| Novogent N |
| Ibutid |
| fenbid |
| 2-(4-Isobutylphenyl)propanoic acid |
| MOTRIN |
| Andran |
| Bluton |
| Dolgirid |
| 4-Isobutyl-α-methylphenylacetic Acid |
| Amibufen |
| MFCD00010393 |
| Nurofen |
| Racemic ibuprofen |
| Advil |
| Brufen |
| Dolo-Dolgit |
| Seclodin |
| UNII:WK2XYI10QM |
| IbU |
| Panafen |
| Adran |
 CAS#:41283-72-1
CAS#:41283-72-1 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:63444-56-4
CAS#:63444-56-4 CAS#:40150-92-3
CAS#:40150-92-3 CAS#:62049-65-4
CAS#:62049-65-4 CAS#:51407-46-6
CAS#:51407-46-6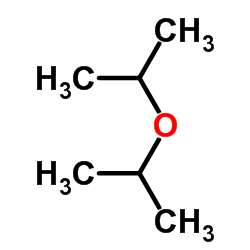 CAS#:108-20-3
CAS#:108-20-3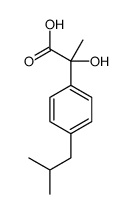 CAS#:60057-62-7
CAS#:60057-62-7 CAS#:36039-36-8
CAS#:36039-36-8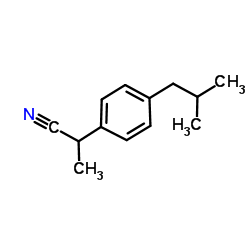 CAS#:58609-73-7
CAS#:58609-73-7![2-[2-[4-(2-methylpropyl)phenyl]propanoylamino]acetic acid structure](https://image.chemsrc.com/caspic/034/110467-58-8.png) CAS#:110467-58-8
CAS#:110467-58-8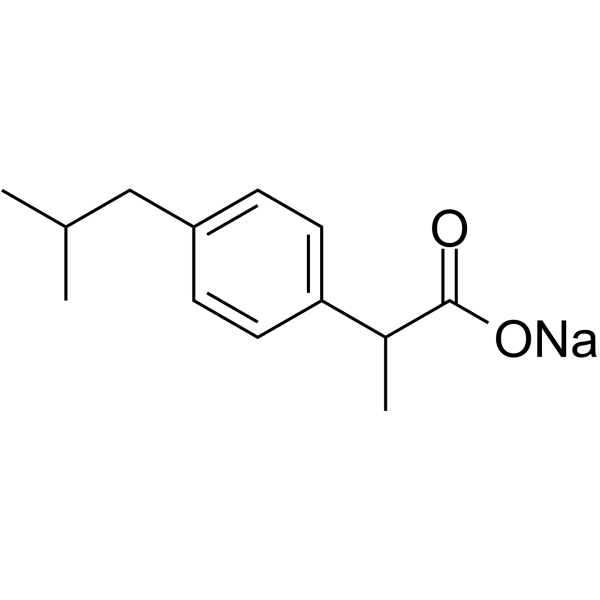 CAS#:31121-93-4
CAS#:31121-93-4 CAS#:40150-98-9
CAS#:40150-98-9 CAS#:38861-78-8
CAS#:38861-78-8 CAS#:43153-07-7
CAS#:43153-07-7 CAS#:100319-40-2
CAS#:100319-40-2 CAS#:65813-55-0
CAS#:65813-55-0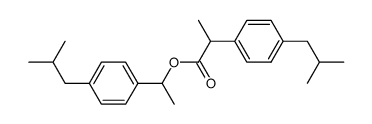 CAS#:69474-20-0
CAS#:69474-20-0
