靛红酸酐
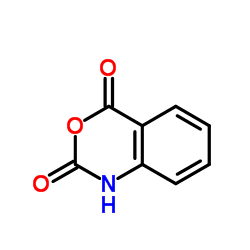
靛红酸酐结构式

|
常用名 | 靛红酸酐 | 英文名 | Isatoic anhydride |
|---|---|---|---|---|
| CAS号 | 118-48-9 | 分子量 | 163.130 | |
| 密度 | 1.4±0.1 g/cm3 | 沸点 | N/A | |
| 分子式 | C8H5NO3 | 熔点 | 233 °C (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 308 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Hydrogen-bonding in 2-aminobenzoyl-alpha-chymotrypsin formed by acylation of the enzyme with isatoic anhydride: IR and mass spectroscopic studies.
ChemBioChem. 3(1) , 68-75, (2002) The acyl-enzyme formed upon acylation of alpha-chymotrypsin with isatoic anhydride has been characterised by infrared spectroscopy. Acylation at pH 7 to yield the 2-aminobenzoyl-enzyme is rapid (k = 5.57x 10(-2)s(-1)), while deacylation is much slower (k =3.7... |
|
|
Palladium-catalyzed decarboxylative coupling of isatoic anhydrides with arylboronic acids.
Org. Lett. 13 , 6114-6117, (2011) The decarboxylative coupling of isatoic anhydrides with arylboronic acids was realized for the first time in the presence of Pd(2)(dba)(3) and DPEphos, achieving aryl o-aminobenzoates with yields ranging from moderate to good. The efficiency of this procedure... |
|
|
1,3-Dipolar cycloaddition-decarboxylation reactions of an azomethine ylide with isatoic anhydrides: formation of novel benzodiazepinones.
Org. Lett. 13(3) , 486-9, (2011) A nonstabilized azomethine ylide reacts with a wide range of substituted isatoic anhydrides to afford novel 1,3-benzodiazepin-5-one derivatives, which are generally isolated in high yield. The transformations involve 1,3-dipolar cycloaddition reactions of the... |
|
|
Suicide enzyme inactivators.
Basic Life Sci. 25 , 287-305, (1983)
|
|
|
Synthesis of 2,3-dihydroquinazolin-4(1H)-ones by three-component coupling of isatoic anhydride, amines, and aldehydes catalyzed by magnetic Fe(3)O(4) nanoparticles in water.
J. Comb. Chem. 12(5) , 643-6, (2010) A simple and efficient protocol for one-pot three-component coupling of isatoic anhydride, amines, and aldehydes in water using magnetically recoverable Fe(3)O(4) nanoparticles is reported. This methodology results in the synthesis of a variety of 2,3-dihydro... |
|
|
Novel and efficient one-pot tandem synthesis of 2-styryl-substituted 4(3H)-quinazolinones.
J. Comb. Chem. 10(5) , 700-3, (2008) A novel one-pot tandem synthesis of 2-styryl-4(3 H)-quinazolinones in an acidic ionic liquid is reported. In this procedure isatoic anhydride, a primary aniline or ammonium acetate, and triethylorthoacetate are reacted in the presence of imidazolium trifluoro... |
|
|
Palladium-catalyzed regioselective carbonylation of C-H bonds of N-alkyl anilines for synthesis of isatoic anhydrides.
J. Am. Chem. Soc. 134(42) , 17490-3, (2012) A Pd-catalyzed regioselective C-H bond carbonylation of N-alkyl anilines for the synthesis of isatoic anhydrides has been developed. The key Pd-catalyst intermediate has been isolated and characterized. This novel Pd-catalyzed carbonylation reaction tolerates... |
|
|
Substituted isatoic anhydrides: selective inactivators of trypsin-like serine proteases.
J. Med. Chem. 29(4) , 585-9, (1986) Derivatives of isatoic anhydride were prepared and tested as inhibitors of serine proteases. A number of isatoic anhydrides with positively charged substituents irreversibly inactivated several trypsin-like enzymes and preferentially inactivated trypsin over ... |
|
|
A new cascade reaction: concurrent construction of six and five membered rings leading to novel fused quinazolinones.
Org. Biomol. Chem. 10(15) , 3098-103, (2012) A one-pot cascade reaction has been developed leading to the concurrent construction of six and five membered fused N-heterocyclic rings of indazolo[3,2-b]quinazolinones. The methodology involved the reaction of isatoic anhydride, a hydrazine and o-iodo benza... |
|
|
Inactivation of arginine esterase E-I of Bitis gabonica venom by irreversible inhibitors including a water-soluble carbodiimide, a chloromethyl ketone and isatoic anhydride.
Int. J. Biochem. 23(10) , 1101-10, (1991) 1. Esterase E-I from Bitis gabonica was inactivated with irreversible inhibitors which included studies with a water-soluble carbodiimide, an affinity labelling peptide and a mechanism-based inactivator. 2. The reaction with 1-ethyl-3(3-dimethylaminopropyl)-c... |