Isatoic anhydride
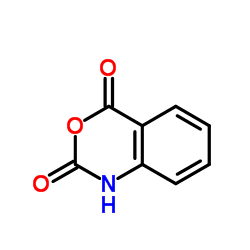
Isatoic anhydride structure
|
Common Name | Isatoic anhydride | ||
|---|---|---|---|---|
| CAS Number | 118-48-9 | Molecular Weight | 163.130 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C8H5NO3 | Melting Point | 233 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 308 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 4H-3,1-Benzoxazine-2,4(1H)-dione |
|---|---|
| Synonym | More Synonyms |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 233 °C (dec.)(lit.) |
| Molecular Formula | C8H5NO3 |
| Molecular Weight | 163.130 |
| Flash Point | 308 °C |
| Exact Mass | 163.026947 |
| PSA | 63.07000 |
| LogP | 0.90 |
| Vapour density | 5.6 (vs air) |
| Index of Refraction | 1.586 |
| Water Solubility | decomposes |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H319 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36;R43 |
| Safety Phrases | S24-S26-S37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | DM3100000 |
| HS Code | 2934999090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Hydrogen-bonding in 2-aminobenzoyl-alpha-chymotrypsin formed by acylation of the enzyme with isatoic anhydride: IR and mass spectroscopic studies.
ChemBioChem. 3(1) , 68-75, (2002) The acyl-enzyme formed upon acylation of alpha-chymotrypsin with isatoic anhydride has been characterised by infrared spectroscopy. Acylation at pH 7 to yield the 2-aminobenzoyl-enzyme is rapid (k = 5... |
|
|
Palladium-catalyzed decarboxylative coupling of isatoic anhydrides with arylboronic acids.
Org. Lett. 13 , 6114-6117, (2011) The decarboxylative coupling of isatoic anhydrides with arylboronic acids was realized for the first time in the presence of Pd(2)(dba)(3) and DPEphos, achieving aryl o-aminobenzoates with yields rang... |
|
|
1,3-Dipolar cycloaddition-decarboxylation reactions of an azomethine ylide with isatoic anhydrides: formation of novel benzodiazepinones.
Org. Lett. 13(3) , 486-9, (2011) A nonstabilized azomethine ylide reacts with a wide range of substituted isatoic anhydrides to afford novel 1,3-benzodiazepin-5-one derivatives, which are generally isolated in high yield. The transfo... |
| 2H-3,1-Benzoxazine-2,4(1H)-dione |
| Anthranilic acid N-carboxylic acid anhydride |
| Isatoic anhydride |
| 3,1-Benzoxazine-2,4(1H)-dione |
| 1H-3,1-benzoxazine-2,4-dione |
| 1H-Benzo[d][1,3]oxazine-2,4-dione |
| Isatoic acid anhydride |
| T66 BMVOVJ |
| Benzoic acid, 2-(carboxyamino)-, cyclic anhydride |
| Benzoxazinedione |
| Anthranilic acid, N-carboxy-, cyclic anhydride |
| MFCD00006700 |
| EINECS 204-255-0 |
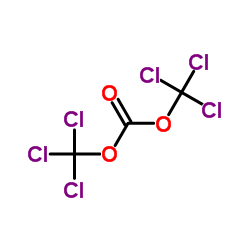 CAS#:32315-10-9
CAS#:32315-10-9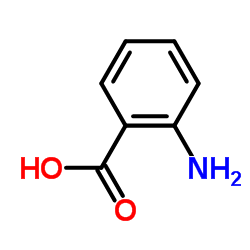 CAS#:118-92-3
CAS#:118-92-3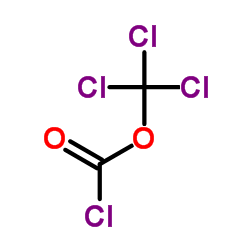 CAS#:503-38-8
CAS#:503-38-8 CAS#:91-56-5
CAS#:91-56-5 CAS#:487-89-8
CAS#:487-89-8 CAS#:120-72-9
CAS#:120-72-9 CAS#:64784-13-0
CAS#:64784-13-0 CAS#:88-97-1
CAS#:88-97-1 CAS#:124-38-9
CAS#:124-38-9 CAS#:10268-69-6
CAS#:10268-69-6 CAS#:106795-52-2
CAS#:106795-52-2 CAS#:110552-32-4
CAS#:110552-32-4![4-[(2-aminobenzoyl)amino]butanoic acid structure](https://image.chemsrc.com/caspic/151/107466-55-7.png) CAS#:107466-55-7
CAS#:107466-55-7 CAS#:10494-82-3
CAS#:10494-82-3![3'-n-butyl-1'H-spiro[indoline-3,2'-quinazoline]-2,4'(3'H)-dione structure](https://image.chemsrc.com/caspic/205/1158178-15-4.png) CAS#:1158178-15-4
CAS#:1158178-15-4 CAS#:105024-96-2
CAS#:105024-96-2 CAS#:36192-63-9
CAS#:36192-63-9 CAS#:35710-05-5
CAS#:35710-05-5 CAS#:607-19-2
CAS#:607-19-2
