Eugenol
Eugenol structure
|
Common Name | Eugenol | ||
|---|---|---|---|---|
| CAS Number | 97-53-0 | Molecular Weight | 164.201 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 255.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H12O2 | Melting Point | −12-−10 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 119.8±8.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of EugenolEugenol is an essential oil found in cloves with antibacterial, anthelmintic and antioxidant activity. Eugenol is shown to inhibit lipid peroxidation. |
| Name | eugenol |
|---|---|
| Synonym | More Synonyms |
| Description | Eugenol is an essential oil found in cloves with antibacterial, anthelmintic and antioxidant activity. Eugenol is shown to inhibit lipid peroxidation. |
|---|---|
| Related Catalog | |
| Target |
Bacterial, Parasite[1] |
| In Vitro | The essential oil of O. gratissimum, as well as eugenol, are efficient in inhibiting eclodibility of H. contortus eggs, showing possible utilizations in the treatment of gastrointestinal helmintosis of small ruminants. At 0.50% concentration, the essential oil and eugenol show a maximum eclodibility inhibition[1]. Eugenol inhibits superoxide anion generation in xanthine-xanthine oxidase system to an extent of 50% at concentrations of 250 μM. Eugenol also inhibits the generation of hydroxyl radicals to an extent of 70%. The OH-radical formation measured by the hydroxylation of salicylate to 2, 3-dihydroxy benzoate is inhibited to an extent of 46% by eugenol at 250 μM[2]. Eugenol protects against RS-induced development of IBS-like gastrointestinal dysfunction through modulation of HPA-axis and brain monoaminergic pathways apart from its antioxidant effect. Eugenol (50 mg/kg) reduces 80% of RS-induced increase in fecal pellets similar to that of ondansetron. Eugenol attenuates 80% of stress-induced increase in plasma corticosterone and modulates the serotonergic system in the PFC and amygdala. Eugenol attenuates stress-induced changes in norepinephrine and potentiates the antioxidant defense system in all brain regions[3]. |
| In Vivo | Eugenol (33 mg/kg) administered orally for 2 days causes significant suppression of knee joint edema, which continues to be significantly reduced at the end of the treatment. After 2 days, eugenol-treated mycobacterial arthritic rats show a marked reduction in paw swelling[4]. |
| Cell Assay | The essential oil and eugenol are diluted in aqueous solution of Tween 20 (0.5%) in the following concentrations: 0.0625, 0.12, 0.25, 0.5 and 1.0% to be used in the egg hatch test[1]. |
| Animal Admin | Rats: The treatment groups of arthritic rats are given either ingwerol (0.33 mL/kg or 33 mg/kg) or eugenol (0.33 mL/kg or 33 mg/kg) orally 1 day prior to the induction of arthritis. This treatment is continued for 26 days on a daily basis. Mycobaterium trated rats receive physiological saline orally[4]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 255.0±0.0 °C at 760 mmHg |
| Melting Point | −12-−10 °C(lit.) |
| Molecular Formula | C10H12O2 |
| Molecular Weight | 164.201 |
| Flash Point | 119.8±8.1 °C |
| Exact Mass | 164.083725 |
| PSA | 29.46000 |
| LogP | 2.20 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.536 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | slightly soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H319 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | SJ4375000 |
| HS Code | 29095090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2909500000 |
|---|---|
| Summary | 2909500000 ether-phenols, ether-alcohol-phenols and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Can Ocimum basilicum L. and Ocimum tenuiflorum L. in vitro culture be a potential source of secondary metabolites?
Food Chem. 194 , 55-60, (2015) In this study Ocimum basilicum L. (OB) and Ocimum tenuiflorum L. (OT) in vitro culture standardisation for increasing eugenol distribution, in comparison to their respective field grown parts was carr... |
|
|
Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Québec (Canada) for Wine Production.
Molecules 20 , 10980-1016, (2015) Developed from crosses between Vitis vinifera and North American Vitis species, interspecific hybrid grape varieties are becoming economically significant in northern areas, where they are now extensi... |
|
|
Effect of essential oils, such as raspberry ketone and its derivatives, on antiandrogenic activity based on in vitro reporter gene assay.
Bioorg. Med. Chem. Lett. 20 , 2111-4, (2010) The effect of essential oils, such as raspberry ketone, on androgen (AR) receptor was investigated using a MDA-kb2 human breast cancer cell line for predicting potential AR activity. Among them, eugen... |
| 4-Allyl-2-methoxyphenol |
| Eugenic acid |
| p-Allylguaiacol |
| 2-methoxy-4-allyl phenol |
| 2-methoxy-4-(prop-2-en-1-yl)phenol |
| Eugenol |
| 2U1R DQ CO1 |
| 4-allylguaiacol |
| 2-methoxy-4-(2-propen-1-yl)phenol |
| p-Eugenol |
| femano.2467 |
| EINECS 202-589-1 |
| 1-allyl-4-hydroxy-3-methoxybenzene |
| 2-Methoxy-4-allylphenol |
| Engenol |
| FA 100 |
| Caryophyllic acid |
| FEMA 2467 |
| Phenol, 2-methoxy-4-(2-propen-1-yl)- |
| MFCD00008654 |
 CAS#:4125-45-5
CAS#:4125-45-5![1-[(4-hydroxy-3-methoxy)phenyl]-3-(trimethylsilyl)propan-1-ol Structure](https://image.chemsrc.com/caspic/305/81391-19-7.png) CAS#:81391-19-7
CAS#:81391-19-7 CAS#:4125-43-3
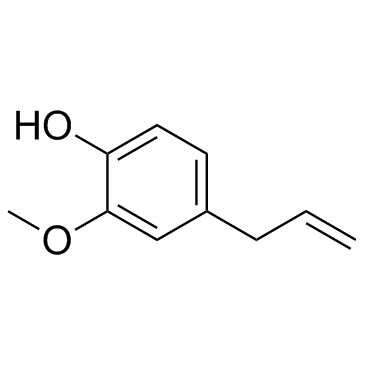
CAS#:4125-43-3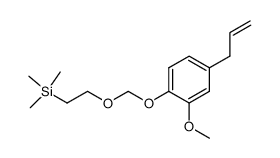 CAS#:76513-64-9
CAS#:76513-64-9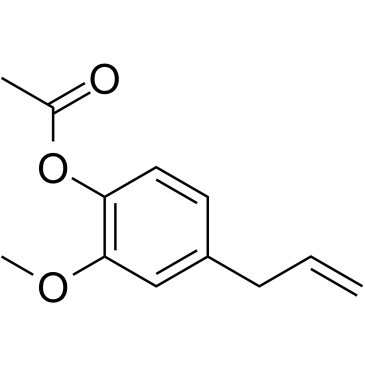 CAS#:93-28-7
CAS#:93-28-7 CAS#:90-05-1
CAS#:90-05-1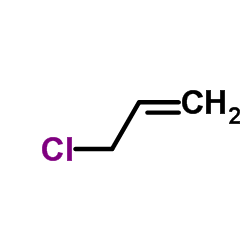 CAS#:107-05-1
CAS#:107-05-1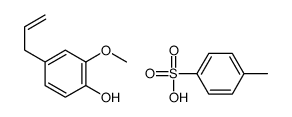 CAS#:144150-79-8
CAS#:144150-79-8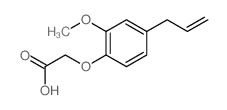 CAS#:6331-61-9
CAS#:6331-61-9 CAS#:110057-59-5
CAS#:110057-59-5![2-[(2-hydroxy-3-methoxy-5-prop-2-enylphenyl)methyl]-6-methoxy-4-prop-2-enylphenol structure](https://image.chemsrc.com/caspic/070/115003-43-5.png) CAS#:115003-43-5
CAS#:115003-43-5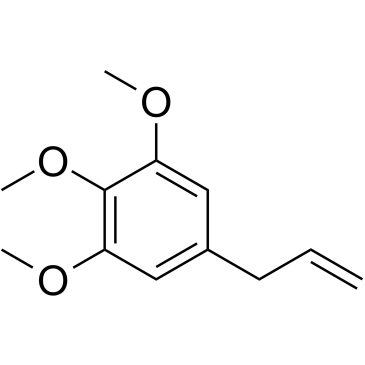 CAS#:487-11-6
CAS#:487-11-6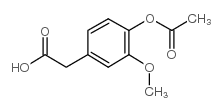 CAS#:5447-38-1
CAS#:5447-38-1 CAS#:607-91-0
CAS#:607-91-0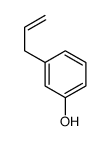 CAS#:1446-24-8
CAS#:1446-24-8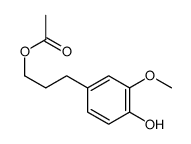 CAS#:14574-06-2
CAS#:14574-06-2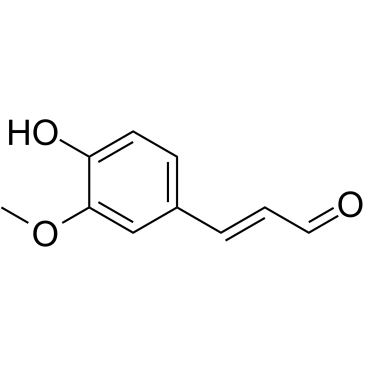 CAS#:458-36-6
CAS#:458-36-6 CAS#:458-35-5
CAS#:458-35-5
