7-Ethyl-10-hydroxycamptothecin
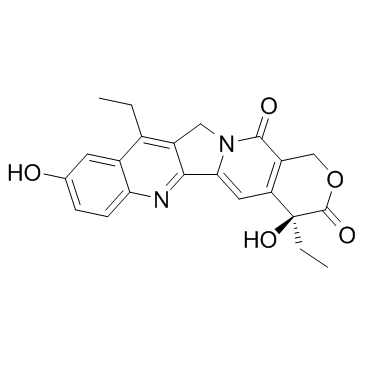
7-Ethyl-10-hydroxycamptothecin structure
|
Common Name | 7-Ethyl-10-hydroxycamptothecin | ||
|---|---|---|---|---|
| CAS Number | 86639-52-3 | Molecular Weight | 392.405 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 810.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C22H20N2O5 | Melting Point | 217 °C | |
| MSDS | Chinese USA | Flash Point | 443.8±34.3 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of 7-Ethyl-10-hydroxycamptothecinSN-38 is an active metabolite of the Topoisomerase I inhibitor Irinotecan. SN-38 inhibits DNA and RNA synthesis with IC50s of 0.077 and 1.3 μM, respectively. |
| Name | 7-Ethyl-10-hydroxycamptothecin |
|---|---|
| Synonym | More Synonyms |
| Description | SN-38 is an active metabolite of the Topoisomerase I inhibitor Irinotecan. SN-38 inhibits DNA and RNA synthesis with IC50s of 0.077 and 1.3 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I |
| In Vitro | The IC50 values for LoVo, HCT116, and HT29 cell lines is 20 nM, 50 nM, 130 nM, respectively. In all three SN-38 resistant cell lines Top1 activity is maintained in the presence of high concentrations of SN-38[2]. |
| In Vivo | SN-38, the active and toxic metabolite of the anticancer prodrug Irinotecan. At 30 minutes after administration, Irinotecan plasma concentrations in Slco1a/1b(−/−) mice are 1.9-fold higher than in the wild-type mice (1.89 vs. 1.01 μM, respectively), whereas SN-38 plasma concentrations of Slco1a/1b(−/−) mice are 8-fold higher compare with wild-type mice (0.4 μg/mL vs. 0.05 μg/mL, respectively). Overall plasma exposure [AUC(5-240)] of Irinotecan is 1.7-fold higher in Oatp1a/1b knockout mice versus wild-type mice (209.8±6.7 vs. 120.9±4.4 μM/min; P<0.01), and 2.9-fold higher for SN-38 (50±2.9 vs. 12±2 μM/min; P<0.001)[3]. |
| Kinase Assay | LoVo, HCT116, and HT29 cell lines are trypsinized, resuspended and counted, and for each cell line 1 million cells are pipetted to each of three eppendorf tubes on ice. Cells are pelleted (5 min centrifugation, 300 g, 4°C) and snap-frozen in dry ice and ethanol and stored at -80°C until analysis. Nuclear extracts are prepared essentially, and Top1 activity measured in titration experiments with or without added SN-38 (at concentrations stated in the text) using the standard Rolling circle Enhanced Enzyme Activity Detection (REEAD) protocol. The activity is calculated in terms of numbers of Top1 specific signals relative to the amount of signals resulting from the addition a known concentration of control circles[2]. |
| Cell Assay | In vitro SN-38 sensitivity is determined using the MTT assay. Cells are seeded in 96-well plates, and a range of SN-38 concentrations is added the following day. Following 48 h of drug exposure, the medium is discarded and the plates are incubated with medium containing MTT (0.5 mg/mL) for 3 h. Acidified (0.02 M HCl) sodium dodecyl sulphate (20 %) is added to dissolve the formed formazan. Optical density at 570 nm (and 670 nm for background) is measured, and the cell viability is calculated in percent compared to untreated cells. Experiments are repeated three times and the mean IC50 value ± standard deviation is determined. Relative resistance for each resistant cell line is calculated by dividing the mean IC50 value of the resistant cell line by the mean IC50 value of the corresponding parental cell line[2]. |
| Animal Admin | Mice[3] Female wild-type, Slco1a/1b(−/−)(Oatp1a/1b knockout), Slco1a/1b(−/−);1B1(tg), and Slco1a/1b(−/−);1B3(tg) (liver-specific OATP1B1 and OATP1B3 humanized transgenic) mice of comparable genetic background (>99% FVB) between 8 and 14 weeks of age are used. Irinotecan (20 mg/mL in water-based solution containing NaOH, lactic acid, and sorbitol) is diluted with saline (to 2 mg/mL) for administration of 10 mg/kg; 5 μL/g bodyweight are administered intravenously to mice. SN-38 is dissolved in DMSO (1 mg/mL) and 1 μL/g body weight is administered intravenously to mice to achieve a dosage of 1 mg/kg. The experiments are terminated by isoflurane anaesthesia, heparin-blood sampling by cardiac puncture followed by cervical dislocation and tissue collection. Blood samples are centrifuged at 5,200 × g for 5 minutes at 4°C and plasma is collected and stored at −30°C until analysis. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 810.3±65.0 °C at 760 mmHg |
| Melting Point | 217 °C |
| Molecular Formula | C22H20N2O5 |
| Molecular Weight | 392.405 |
| Flash Point | 443.8±34.3 °C |
| Exact Mass | 392.137207 |
| PSA | 101.65000 |
| LogP | 2.31 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.738 |
| InChIKey | FJHBVJOVLFPMQE-QFIPXVFZSA-N |
| SMILES | CCc1c2c(nc3ccc(O)cc13)-c1cc3c(c(=O)n1C2)COC(=O)C3(O)CC |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Precursor 8 | |
|---|---|
| DownStream 4 | |
| HS Code | 2942000000 |
|---|
|
Evaluation of the in vitro/in vivo potential of five berries (bilberry, blueberry, cranberry, elderberry, and raspberry ketones) commonly used as herbal supplements to inhibit uridine diphospho-glucuronosyltransferase.
Food Chem. Toxicol. 72 , 13-9, (2014) In this study, we evaluated inhibitory potentials of popularly-consumed berries (bilberry, blueberry, cranberry, elderberry, and raspberry ketones) as herbal supplements on UGT1A1, UGT1A4, UGT1A6, UGT... |
|
|
Evaluation of thein vitro/in vivodrug interaction potential of BST204, a purified dry extract of ginseng, and its four bioactive ginsenosides through cytochrome P450 inhibition/induction and UDP-glucuronosyltransferase inhibition
Food Chem. Toxicol. 68 , 117-27, (2014) • BST204 is a purified dry extract of ginseng containing high amounts of Rh2 and Rg3. • BST204 had only weak inhibitory effects on nine CYPs and five UGTs. • It is unlikely that BST204 alter pharmacok... |
|
|
Nanoparticle delivery of an SN38 conjugate is more effective than irinotecan in a mouse model of neuroblastoma.
Cancer Lett. 360(2) , 205-12, (2015) Neuroblastoma (NB) is the most common and deadly solid tumor in children. The majority of NB patients have advanced stage disease with poor prognosis, so more effective, less toxic therapy is needed. ... |
| 7-Ethyl-10-Hydroxycamptothecin(SN-38) |
| (4S)-4,11-Diethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4,11-diethyl-4,9-dihydroxy-, (4S)- |
| SN-38 |
| MFCD06762720 |
| SN38 |
| 7-Ethyl-10-hydroxycamptothecine |
 CAS#:78287-27-1
CAS#:78287-27-1 CAS#:35364-15-9
CAS#:35364-15-9![(S)-4-ethyl-4-hydroxy-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione Structure](https://image.chemsrc.com/caspic/225/110351-94-5.png) CAS#:110351-94-5
CAS#:110351-94-5 CAS#:797762-11-9
CAS#:797762-11-9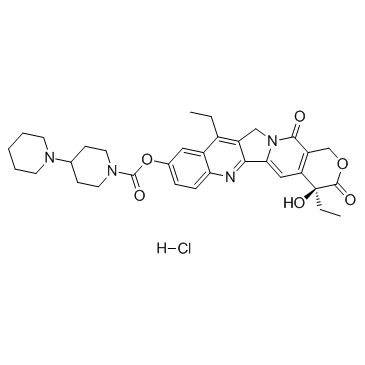 CAS#:100286-90-6
CAS#:100286-90-6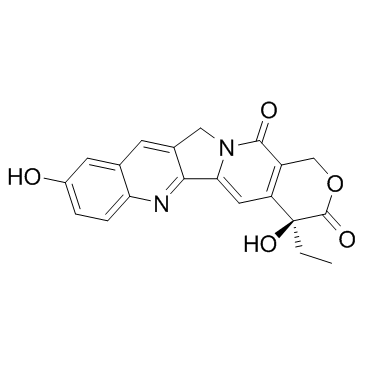 CAS#:19685-09-7
CAS#:19685-09-7 CAS#:123-38-6
CAS#:123-38-6![(±)- 1H-Pyrano[3,4-f]indolizine-3,6,10(4H)-trione, 4-ethyl-7,8-dihydro-4-hydroxy Structure](https://image.chemsrc.com/caspic/342/102978-40-5.png) CAS#:102978-40-5
CAS#:102978-40-5 CAS#:97682-44-5
CAS#:97682-44-5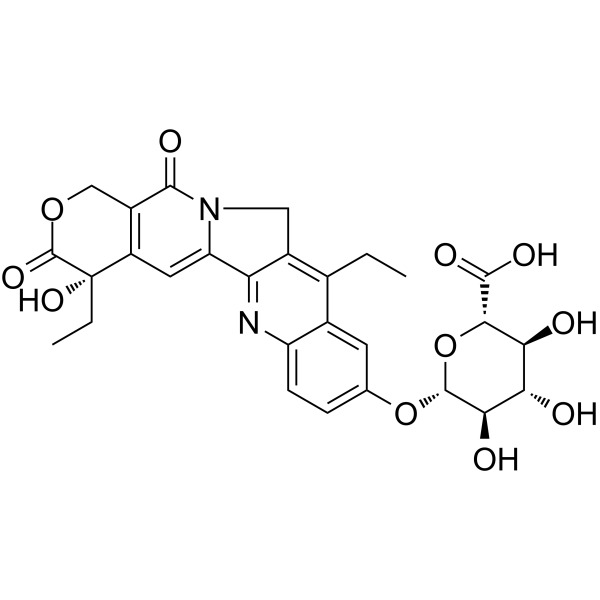 CAS#:121080-63-5
CAS#:121080-63-5 CAS#:121098-77-9
CAS#:121098-77-9
