(S)-10-Hydroxycamptothecin
Modify Date: 2025-08-22 19:30:38
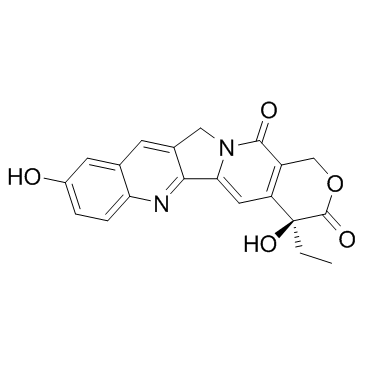
(S)-10-Hydroxycamptothecin structure
|
Common Name | (S)-10-Hydroxycamptothecin | ||
|---|---|---|---|---|
| CAS Number | 19685-09-7 | Molecular Weight | 364.351 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 820.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C20H16N2O5 | Melting Point | 265-270°C | |
| MSDS | N/A | Flash Point | 450.1±34.3 °C | |
Use of (S)-10-Hydroxycamptothecin(S)-10-Hydroxycamptothecin is a clinical therapy agent against hepatoma.IC50 value:Target:In vitro: In vitro, the 10-hydroxycamptothecin nanosuspensions released the encapsulated drug with nearly zero-order kinetics, and the accumulative release reached 90% within 72 hours. In vitro cytotoxicity assay showed that the 10-hydroxycamptothecin nanosuspensions had significantly enhanced cytotoxicity against HepG2 cells compared to the commercially available 10-hydroxycamptothecin injections [1].In vivo: The in vivo study with H22 tumor-bearing mice and intravenous injection of the drug showed that in contrast to the 10-hydroxycamptothecin injections, the 10-hydroxycamptothecin nanosuspensions exhibited significantly enhanced biodistribution, particularly in the lung (393.40-fold AUC0–24 h, liver (192.35-fold AUC0–24 h, spleen (141.67-fold AUC0–24 h and tumor (64.21-fold AUC0–24 h. The 10-hydroxycamptothecin nanosuspensions also showed improved antitumor therapeutic efficacy over the injections (89.83% vs. 30.56%) [1]. |
| Name | 10-Hydroxycamptothecin |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-10-Hydroxycamptothecin is a clinical therapy agent against hepatoma.IC50 value:Target:In vitro: In vitro, the 10-hydroxycamptothecin nanosuspensions released the encapsulated drug with nearly zero-order kinetics, and the accumulative release reached 90% within 72 hours. In vitro cytotoxicity assay showed that the 10-hydroxycamptothecin nanosuspensions had significantly enhanced cytotoxicity against HepG2 cells compared to the commercially available 10-hydroxycamptothecin injections [1].In vivo: The in vivo study with H22 tumor-bearing mice and intravenous injection of the drug showed that in contrast to the 10-hydroxycamptothecin injections, the 10-hydroxycamptothecin nanosuspensions exhibited significantly enhanced biodistribution, particularly in the lung (393.40-fold AUC0–24 h, liver (192.35-fold AUC0–24 h, spleen (141.67-fold AUC0–24 h and tumor (64.21-fold AUC0–24 h. The 10-hydroxycamptothecin nanosuspensions also showed improved antitumor therapeutic efficacy over the injections (89.83% vs. 30.56%) [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 820.7±65.0 °C at 760 mmHg |
| Melting Point | 265-270°C |
| Molecular Formula | C20H16N2O5 |
| Molecular Weight | 364.351 |
| Flash Point | 450.1±34.3 °C |
| Exact Mass | 364.105927 |
| PSA | 101.65000 |
| LogP | 1.32 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.777 |
| InChIKey | HAWSQZCWOQZXHI-FQEVSTJZSA-N |
| SMILES | CCC1(O)C(=O)OCc2c1cc1n(c2=O)Cc2cc3cc(O)ccc3nc2-1 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 2934999090 |
| Precursor 7 | |
|---|---|
| DownStream 7 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 10-Hydroxycamptothecin |
| 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4,9-dihydroxy-, (4S)- |
| Hydroxycamptothecine |
| 10-Hydroxy camptothecin |
| 10-OH-camptothecin |
| 10-hydroxy-CPT |
| Camptothecin,hydroxy |
| MFCD00189425 |
| 10-Hydroxycamptochecin |
| Camptothecin, 10-hydroxy |
| 10-hydroxy-Camptothecin |
| (4S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| Hydroxycamptothecin |
| 10-hydroxycamptothecine |
| Irinotecan Related Compound A |
| HCPT |
| (S)-10-Hydroxycamptothecin |
 CAS#:7689-03-4
CAS#:7689-03-4![(4S)-4-Ethyl-4-hydroxy-9-methoxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione Structure](https://image.chemsrc.com/caspic/000/19685-10-0.png) CAS#:19685-10-0
CAS#:19685-10-0 CAS#:870527-52-9
CAS#:870527-52-9![(S)-10-((Dimethylamino)methyl)-4-ethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl acetate Structure](https://image.chemsrc.com/caspic/478/123948-88-9.png) CAS#:123948-88-9
CAS#:123948-88-9![(S)-4-ethyl-4-hydroxy-6-iodo-3-oxo-1H-pyrano[3,4-c]-8-pyridone Structure](https://image.chemsrc.com/caspic/163/173442-34-7.png) CAS#:173442-34-7
CAS#:173442-34-7 CAS#:86639-48-7
CAS#:86639-48-7 CAS#:623-51-8
CAS#:623-51-8 CAS#:91421-42-0
CAS#:91421-42-0 CAS#:91421-43-1
CAS#:91421-43-1 CAS#:86639-52-3
CAS#:86639-52-3 CAS#:104267-73-4
CAS#:104267-73-4 CAS#:123948-87-8
CAS#:123948-87-8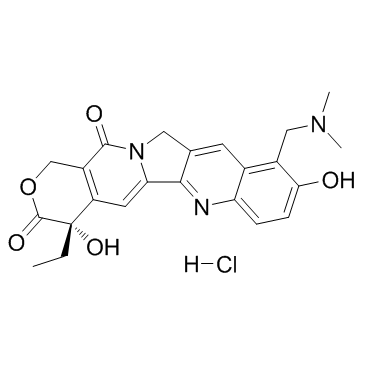 CAS#:119413-54-6
CAS#:119413-54-6
