6-Aminopenicillanic acid
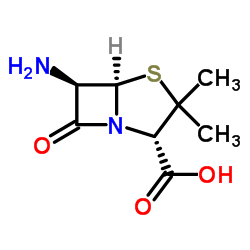
6-Aminopenicillanic acid structure
|
Common Name | 6-Aminopenicillanic acid | ||
|---|---|---|---|---|
| CAS Number | 551-16-6 | Molecular Weight | 216.258 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 460.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C8H12N2O3S | Melting Point | 198-200 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 232.1±28.7 °C | |
Use of 6-Aminopenicillanic acid6-Aminopenicillanic acid (6-APA) is an important precursor for the synthesis of -lactam antibiotics. 6-Aminopenicillanic acid is the main product of Penicillin G (PenG) hydrolyzed by penicillin acylase (PA)[1]. |
| Name | 6-aminopenicillanic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Aminopenicillanic acid (6-APA) is an important precursor for the synthesis of -lactam antibiotics. 6-Aminopenicillanic acid is the main product of Penicillin G (PenG) hydrolyzed by penicillin acylase (PA)[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 460.2±45.0 °C at 760 mmHg |
| Melting Point | 198-200 °C (dec.)(lit.) |
| Molecular Formula | C8H12N2O3S |
| Molecular Weight | 216.258 |
| Flash Point | 232.1±28.7 °C |
| Exact Mass | 216.056870 |
| PSA | 108.93000 |
| LogP | -0.36 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.656 |
| Storage condition | 2~8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
|---|---|
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R42/43 |
| Safety Phrases | S22-S36/37-S45-S37-S24 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | XH8225000 |
| HS Code | 2941109300 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2941109300 |
|---|---|
| Summary | 2941109300. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:Q(report of inspection of soundness on import medicines). MFN tariff:4.0%. General tariff:20.0% |
|
Enhanced production of 6-aminopenicillanic acid in aqueous methyl isobutyl ketone system with immobilized penicillin G acylase.
Prep Biochem Biotechnol. 40(1) , 38-45, (2010) Enzymatic hydrolysis of penicillin G for production of 6-amino-penicillanic-acid (6-APA) was achieved by using penicillin G acylase as catalyst in an aqueous-methylisobutyl ketone (MIBK) system. The o... |
|
|
Monitoring bioreactors using principal component analysis: production of penicillin G acylase as a case study.
Bioprocess Biosyst. Eng. 33(5) , 557-64, (2010) The complexity of biological processes often makes impractical the development of detailed, structured phenomenological models of the cultivation of microorganisms in bioreactors. In this context, dat... |
|
|
Synthesis and biological evaluation of penem inhibitors of bacterial signal peptidase.
Bioorg. Med. Chem. Lett. 19(14) , 3787-90, (2009) We report the first synthesis of a 5S penem, known to bind bacterial type I signal peptidase, from the commercially available and inexpensive 6-aminopenicillanic acid. We report the first in vivo acti... |
| [2S-(2a,5a,6b)]-6-Amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid |
| EINECS 208-993-4 |
| 6-aps |
| 6-AMINO PENICILLINIC ACID |
| 6-Aminopenicillanic acid |
| penicin |
| 6-Aminopenicillansure |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-amino-3,3-dimethyl-7-oxo-, (2S,5R,6R)- |
| aminopenicillanicacid |
| MFCD00005176 |
| 6-APA |
| 6b-Aminopenicillanic Acid |
| (2S,5R,6R)-6-amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| Amoxicillin Impurity 1 |
 CAS#:87-08-1
CAS#:87-08-1![(2S,5R,6R)-6-(1,3-dioxoisoindol-2-yl)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Structure](https://image.chemsrc.com/caspic/136/20425-27-8.png) CAS#:20425-27-8
CAS#:20425-27-8![(2S,5R,6S)-Benzyl 3,3-Dimethyl-7-oxo-6-[[(trifluoromethyl)sulfonyl]oxy]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate Structure](https://image.chemsrc.com/caspic/101/128971-96-0.png) CAS#:128971-96-0
CAS#:128971-96-0![(3S,4R)-3-[(tert-Butyldiphenylsilyl)oxy]-1-[(2,3-dimethyl-2-butyl)dimethylsilyl]-4-[(2-methyl-3-oxo-2-propyl)thio]-2-azetidinone Structure](https://image.chemsrc.com/caspic/263/128971-92-6.png) CAS#:128971-92-6
CAS#:128971-92-6![(3S,4R)-3-[(tert-Butyldiphenylsilyl)oxy]-1-[(2,3-dimethyl-2-butyl)dimethylsilyl]-4-[[4-(benzyloxy)-3-hydroxy-2-methyl-4-nitro-2-butyl]thio]-2-azetidinone Structure](https://image.chemsrc.com/caspic/225/128971-93-7.png) CAS#:128971-93-7
CAS#:128971-93-7 CAS#:37091-66-0
CAS#:37091-66-0 CAS#:32887-01-7
CAS#:32887-01-7 CAS#:14796-35-1
CAS#:14796-35-1 CAS#:19379-33-0
CAS#:19379-33-0 CAS#:69-53-4
CAS#:69-53-4 CAS#:2935-35-5
CAS#:2935-35-5![(2S)-6,6-dibromo-3,3-dimethyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid structure](https://image.chemsrc.com/caspic/215/205320-24-7.png) CAS#:205320-24-7
CAS#:205320-24-7![methyl 3,3-dimethyl-7-oxo-6-[(2-phenoxyacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate structure](https://image.chemsrc.com/caspic/137/2315-05-1.png) CAS#:2315-05-1
CAS#:2315-05-1 CAS#:343-55-5
CAS#:343-55-5 CAS#:61-33-6
CAS#:61-33-6
