| Structure | Name/CAS No. | Articles |
|---|---|---|
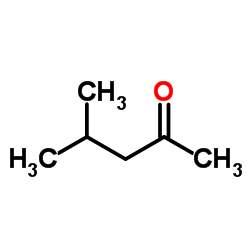 |
4-Methyl-2-pentanone
CAS:108-10-1 |
|
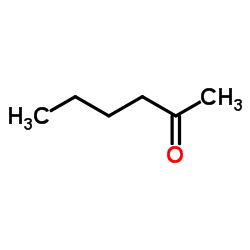 |
2-Hexanone
CAS:591-78-6 |
|
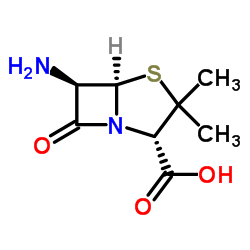 |
6-Aminopenicillanic acid
CAS:551-16-6 |