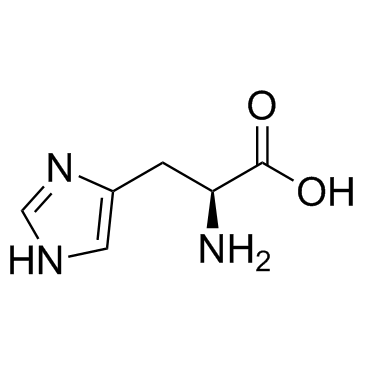
Gly-His

Gly-His structure
|
Common Name | Gly-His | ||
|---|---|---|---|---|
| CAS Number | 2489-13-6 | Molecular Weight | 212.20600 | |
| Density | 1.56g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C8H12N4O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Gly-His(S)-2-(2-Aminoacetamido)-3-(1H-imidazol-4-yl)propanoic acid is a histidine derivative[1]. |
| Name | h-gly-his-oh |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-2-(2-Aminoacetamido)-3-(1H-imidazol-4-yl)propanoic acid is a histidine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.56g/cm3 |
|---|---|
| Molecular Formula | C8H12N4O3 |
| Molecular Weight | 212.20600 |
| Exact Mass | 212.09100 |
| PSA | 121.10000 |
| Vapour Pressure | 3.33E-18mmHg at 25°C |
| Index of Refraction | 1.604 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| HS Code | 2933290090 |
|
~82% 
Gly-His CAS#:2489-13-6 |
| Literature: Chemische Berichte, , vol. 128, # 6 p. 541 - 550 |
|
~% 
Gly-His CAS#:2489-13-6 |
| Literature: Journal of the American Chemical Society, , vol. 75, p. 2388 |
|
~% 
Gly-His CAS#:2489-13-6 |
| Literature: Journal of the American Chemical Society, , vol. 75, p. 2388 |
|
~% 
Gly-His CAS#:2489-13-6 |
| Literature: Chemistry - A European Journal, , vol. 14, # 8 p. 2536 - 2541 |
|
~% 
Gly-His CAS#:2489-13-6 |
| Literature: Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, , vol. 307, p. 23,31 |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Effect of carnosine and related compounds on the inactivation of human Cu,Zn-superoxide dismutase by modification of fructose and glycolaldehyde.
Biosci. Biotechnol. Biochem. 66 , 36-43, (2002) Glycolaldehyde, an intermediate of the Maillard reaction, and fructose, which is mainly derived from the polyol pathway, rapidly inactivate human Cu,Zn-superoxide dismutase (SOD) at the physiological ... |
|
|
Reduction of vanadium(V) to vanadium(IV) by NADPH, and vanadium(IV) to vanadium(III) by cysteine methyl ester in the presence of biologically relevant ligands.
Biochim. Biophys. Acta 1770(8) , 1212-8, (2007) To better understand the mechanism of vanadium reduction in ascidians, we examined the reduction of vanadium(V) to vanadium(IV) by NADPH and the reduction of vanadium(IV) to vanadium(III) by L-cystein... |
|
|
A comparative study of complex formation in the reactions of gold(III) with Gly-Gly, Gly-L-Ala and Gly-L-His dipeptides.
Bioorg. Chem. 38(4) , 144-8, (2010) Proton NMR spectroscopy was applied to study the reactions of the dipeptides glycyl-glycine (Gly-Gly) and glycyl-L-alanine (Gly-L-Ala) with hydrogen tetrachloridoaurate(III) (H[AuCl(4)]). All reaction... |
| GLY-HIS |
| N-Glycyl-L-histidine |
| Gly-L-His-L-Lis-OH |
| L-Gly-L-His-OH |
| Gly-L-His-OH |
| gly-his free base |
| glycylhistidine |
| Gly-His-OH |
| (2S)-2-(glycylamino)-3-(4H-imidazol-4-yl)propionic acid |
| glycyl-L-histidine |
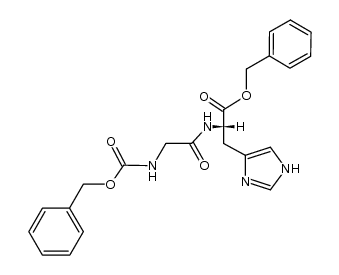
![ethyl 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopyridin-1(2H)-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylate structure](https://image.chemsrc.com/caspic/495/503622-97-7.png)


 CAS#:56-40-6
CAS#:56-40-6
