Rosmarinic acid
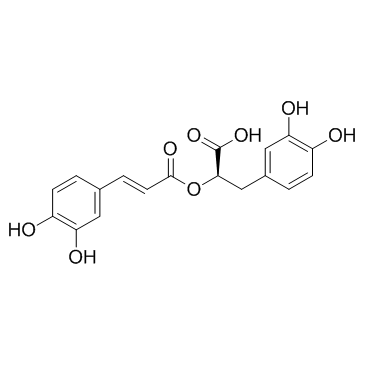
Rosmarinic acid structure
|
Common Name | Rosmarinic acid | ||
|---|---|---|---|---|
| CAS Number | 20283-92-5 | Molecular Weight | 360.315 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 694.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C18H16O8 | Melting Point | 171-175 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 254.5±25.0 °C | |
| Symbol |


GHS06, GHS09 |
Signal Word | Danger | |
Use of Rosmarinic acidRosmarinic acid (RA) is a widespread phenolic ester compound in the plants. Rosmarinic acid inhibits MAO-A, MAO-B and COMT enzymes with IC50s of 50.1, 184.6 and 26.7 μM, respectively. |
| Name | rosmarinic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Rosmarinic acid (RA) is a widespread phenolic ester compound in the plants. Rosmarinic acid inhibits MAO-A, MAO-B and COMT enzymes with IC50s of 50.1, 184.6 and 26.7 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 50.1 μM (MAO-A), 184.6 μM (MAO-B), 26.7 μM (COMT)[1] |
| In Vitro | Rosmarinic acid (RA) shows an in vitro multifunctional profile characterized by antioxidant effects, and monoamine oxidases (MAO-A and MAO-B) and catechol-O-methyl transferase (COMT) inhibition. Rosmarinic acid shows antioxidant effects against hydroxyl (HO(•)) and nitric oxide (NO) radicals (IC50 of 29.4 and 140 μM, respectively), and inhibition of lipid peroxidation (IC50 of 19.6 μM)[1]. Rosmarinic acid (RA) exerts a significant cytoprotective effect by scavenging intracellular ROS induced by UVB. In H2O2-treated cells, 2.5 μM Rosmarinic acid scavenges 60% of intracellular ROS compared to 77% of intracellular ROS scavenging effect in N-acetyl-L-cysteine (NAC)[2]. |
| In Vivo | Rosmarinic acid (RA) is a widespread phenolic ester compound in the plants, particularly those in the Labiatae family of herbs, such as Rosmarinus officinali, Salvia miltiorrhiza, and Prunella vulgaris. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. In the DSS-induced colitis model, Treatment with Rosmarinic acid (30, 60 mg/kg, p.o.) markedly attenuates the production of cytokines[3]. |
| Cell Assay | Human keratinocytes (HaCaT cells) are treated with Rosmarinic acid (0.625, 1.25, 2.5, or 5 μM) and exposed to UVB radiation 1 h later. They are then incubated at 37°C for 48 h. At this time, MTT is added to each well to obtain a total reaction volume of 200 μL. After 4 h incubation, the supernatant is removed by aspiration. The formazan crystals in each well are dissolved in dimethyl sulfoxide (DMSO; 150 μL), and the absorbance at 540 nm is measured on a scanning multi-well spectrophotometer[2]. |
| Animal Admin | Mice[3] Experimental colitis is induced by giving mice drinking water ad libitum containing 5% (w/v) DSS for 7 days. Mice of each of the groups are monitored carefully every day to confirm that they have consumed an approximately equal volume of water containing DSS. For each experiment, the mice are divided into five experimental groups (n = 10/group). The first group is kept as the vehicle-treated control, and the second group is given drinking water with DSS only during the experimental period. The other three groups consist of mice receiving 5% DSS who are administrated 5-ASA (75 mg/kg/day p.o.) or Rosmarinic acid (30 or 60 mg/kg/day p.o.) daily for 7 days. Control groups are given the vehicle daily for 7 days as appropriate. Administration of each drug is initiated simultaneously with the DSS treatment. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 694.7±55.0 °C at 760 mmHg |
| Melting Point | 171-175 °C(lit.) |
| Molecular Formula | C18H16O8 |
| Molecular Weight | 360.315 |
| Flash Point | 254.5±25.0 °C |
| Exact Mass | 360.084503 |
| PSA | 144.52000 |
| LogP | 1.70 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.714 |
| InChIKey | DOUMFZQKYFQNTF-WUTVXBCWSA-N |
| SMILES | O=C(C=Cc1ccc(O)c(O)c1)OC(Cc1ccc(O)c(O)c1)C(=O)O |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H400 |
| Precautionary Statements | P273-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi |
| Risk Phrases | 36/38 |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | GD8990000 |
| HS Code | 2932999099 |
| Precursor 7 | |
|---|---|
| DownStream 4 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Methanolic extract of Origanum vulgare ameliorates type 1 diabetes through antioxidant, anti-inflammatory and anti-apoptotic activity.
Br. J. Nutr. 113(5) , 770-82, (2015) Type 1 diabetes (T1D), an autoimmune inflammatory disorder, develops as a consequence of pancreatic β-cell destruction and results in hyperglycaemia. Since current T1D therapy mainly involves insulin ... |
|
|
The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage.
Food Chem. Toxicol. 55 , 92-9, (2013) Perilla frutescens leaves are often used in East Asian gourmet food. In this study, we investigated the hepatoprotective effects of caffeic acid (CA), rosmarinic acid (RA), and their combination. P. f... |
|
|
Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts.
Food Chem. 161 , 79-86, (2014) Antimicrobial properties of ethanol and water extracts from eight Asteraceae species were investigated against three Gram positive (Staphylococcus aureus, MRSA and Bacillus cereus) and two Gram negati... |
| R-(+)-2-(3,4-Dihydroxycinnamoyloxy)-3-(3,4-dihydroxyphenyl)propionic acid |
| MFCD00017740 |
| ROSMARINATE |
| (R)-rosmarinic acid |
| (+)-Rosmarinic acid |
| Benzenepropanoic acid, α-[[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl]oxy]-3,4-dihydroxy-, (αR)- |
| Rosemary acid |
| (2R)-3-(3,4-Dihydroxyphenyl)-2-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}propanoic acid |
| (2R)-3-(3,4-Dihydroxyphenyl)-2-{[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]oxy}propanoic acid |
| RosmarinicAcid |
| Rosmarinic acid |
| rosemaryacid |
| Rosmarinic |
| Rosemaricacid |
| Labiatenic acid |
| 3,4-Dihydroxycinnamicacid(R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethylester |
| (R)-O-(3,4-Dihydroxycinnamoyl)-3-(3,4-dihydroxyphenyl)lacticacid |
| 4-dihydroxy |
 CAS#:179462-78-3
CAS#:179462-78-3 CAS#:179462-77-2
CAS#:179462-77-2 CAS#:68307-79-9
CAS#:68307-79-9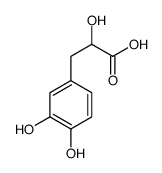 CAS#:23028-17-3
CAS#:23028-17-3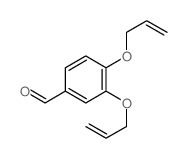 CAS#:71186-67-9
CAS#:71186-67-9 CAS#:179128-86-0
CAS#:179128-86-0![(R,E)-1-(allyloxy)-3-(benzo[d][1,3]dioxol-5-yl)-1-oxopropan-2-yl 3-(3,4-bis(benzyloxy)phenyl)acrylate Structure](https://image.chemsrc.com/caspic/498/179129-01-2.png) CAS#:179129-01-2
CAS#:179129-01-2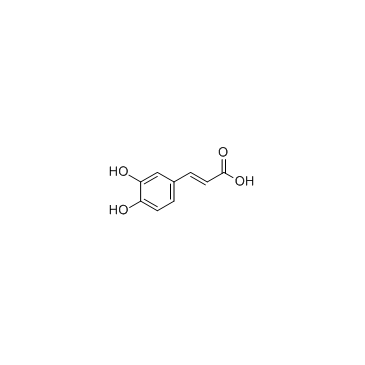 CAS#:331-39-5
CAS#:331-39-5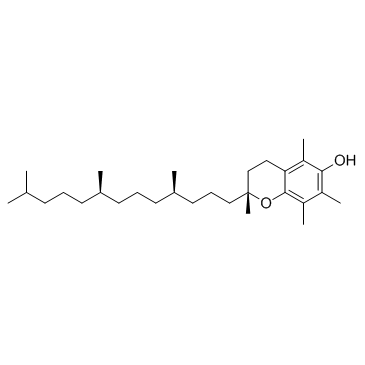 CAS#:59-02-9
CAS#:59-02-9![methyl 3-[3,4-bis(phenylmethoxy)phenyl]prop-2-enoate structure](https://image.chemsrc.com/caspic/104/71038-25-0.png) CAS#:71038-25-0
CAS#:71038-25-0
