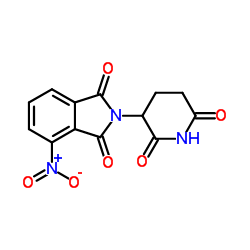
Pomalidomide
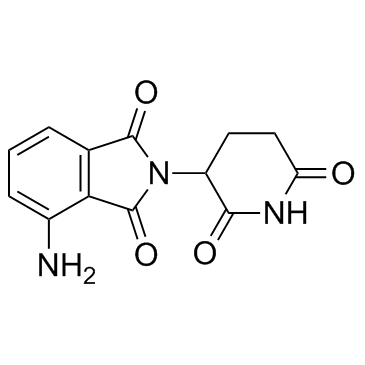
Pomalidomide structure
|
Common Name | Pomalidomide | ||
|---|---|---|---|---|
| CAS Number | 19171-19-8 | Molecular Weight | 273.244 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 582.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C13H11N3O4 | Melting Point | 318.5 - 320.5° | |
| MSDS | Chinese USA | Flash Point | 306.3±28.7 °C | |
Use of PomalidomidePomalidomide is an anti-angiogenic agent and an immunomodulator. Pomalidomide inhibits TNF-α release in LPS stimulated human PBMC with an IC50 of 13 nM. |
| Name | pomalidomide |
|---|---|
| Synonym | More Synonyms |
| Description | Pomalidomide is an anti-angiogenic agent and an immunomodulator. Pomalidomide inhibits TNF-α release in LPS stimulated human PBMC with an IC50 of 13 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 13 nM (TNF-α, in PBMCs)[1] |
| In Vitro | Pomalidomide also inhibits Whole Blood TNF-α with IC50 of 25 nM[1]. Exposure of lymphoma cells to Pomalidomide (CC-4047) leads to 40% decrease in cell proliferation when compared with vehicle-treated controls. Pomalidomide inhibits by 40% the DNA synthesis of Raji cells (P=0.036)[2]. In both CD4+ and CD8+ cells, Pomalidomide (CC-4047) is the most potent IL-2-elevator, followed by CC-6032 and CC-5013. Pomalidomide is significantly more potent than CC-5013 at elevating IL-2, IL-5, and IL-10, and slightly more potent than CC-5013 at elevating IFN-γ[3]. |
| In Vivo | The administration of Pomalidomide (CC-4047) for two consecutive days before mAb therapy enhances the antitumor activity of Rituximab and doubled the median survival of lymphoma-bearing mice. Statistically, significant differences are observed between animals treated with Rituximab versus Pomalidomide+Rituximab. The median survival time of animals treated with Pomalidomide and Rituximab is longer (median survival, 74 days; 95% CI, 70-78) than those treated with Rituximab monotherapy (median survival, 38 days; 95% CI, 26-50; log-rank test, P=0.002). The administration of CC-5013 or Pomalidomide for two consecutive days leads to a significant increase in the number of circulating NK cells as shown by flow cytometry analysis, in lymphoma-bearing SCID mice[2]. Following a 50 mg/kg PO administration of Pomalidomide (POM) to rats, unbound concentrations in blood reach a Cmax value of 1100±82 ng/mL at 4.6±2.4 hours, with a concomitant AUC(0-10) value of 6800±2000 ng•hr/mL. Unbound POM in the brain, however, has a Cmax value of 430±63 ng/mL at 4.1±1.5 hours and an AUC(0-10) value of 2700±740 ng•hr/mL, giving an unbound AUCbrain to AUCblood ratio of 0.39±0.03. These values are consistent with excellent blood-brain-barrier penetration. The results obtained in this study are consistent with those seen in a concurrent study looking at whole brain POM content following its oral administration to mice[4]. |
| Cell Assay | Lymphoma cell lines are placed in 96-well plates (1×105 cells per well) and exposed to escalating concentrations of CC-5013, Pomalidomide (2.5, 5, 10, 20, and 40 μg/mL), or vehicle control single agents or in combination with Rituximab or Trastuzumab (isotype), at a final antibody concentration of 10 μg/mL. The final concentration is adjusted to 200 μL with 10% RPMI. The cell lines are incubated at 37°C and 5% CO2 for 24 and 48 hours. Following 24 or 48 hours, 1 μCi per well of [3H]-thymidine is added and cells are incubated for 18 hours more. Cells are then harvested using the Harvest system into the 96-well glass filters and [3H]-thymidine uptake is measured using an automated scintillation counter. Each experiment is done in triplicate at three different times; results are presented as the mean of counts per minute (cpm) at 24 and 48 hours±SD[2]. |
| Animal Admin | Mice[2] Six- to 8-week-old SCID mice are used for this purpose. On day 0, all the animals receive 1×106 Raji cells via tail vein injection. After 72 hours of tumor engraftment, the animals are divided into seven cohorts. The first cohort (group A) serve as control and receive no treatment. Groups B and C consist of animals treated with either CC-5013 (0.5 mg/kg) or Pomalidomide (0.5 mg/kg) given i.p. on days +3, +4, +8, +9, +13, +14, +18, and +19. Groups D and E are treated with Rituximab or Trastuzumab (isotype control) monotherapy given via tail vein injection at 10 mg/kg on days +5, +10, +15, and +20. Finally, groups F and G consist of animals treated with Rituximab in combination with CC-5013 (group E) or Pomalidomide (group G). IMiDs are given i.p. for two consecutive days before each dose of Rituximab. After completion of therapy, animals are observed for a period of 90 days. The end point of the study is survival defined as the time for the development of limb paralysis. Animals that reach the end point or survived after 3 months of observation are sacrificed by cervical dislocation. Pathologic examination of all organs (liver, lung, and brain) is done to detect any residual disease. The experiments are repeated in three separate occasions. Rats[4] A total of 3 male CD-IGS rats are used. Pomalidomide is administered as a single PO administration via the stomach cannula, at 50 mg/kg (5 mL/kg) in a 0.5% carboxymethylcellulose/0.25% Tween 80 suspension formulation. Microdialysate is collected in a cooling fraction collector, set at 4°C at intervals of 25 minutes for 10 hours after dosing. To calculate AUC, the corrected concentration of each sample is multiplied by the interval over which the sample is collected; in this case 25 minutes, and divided by 60 minutes per hour. The sum of these values represented the total AUC value over the specified time range. To generate graphs, the concentration at each time point is plotted at the mid-point of each collection interval. Microdialysates are collected at the specified time points and analyzed for Pomalidomide concentration using a LC-MS/MS assay, within 12 hours. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 582.9±45.0 °C at 760 mmHg |
| Melting Point | 318.5 - 320.5° |
| Molecular Formula | C13H11N3O4 |
| Molecular Weight | 273.244 |
| Flash Point | 306.3±28.7 °C |
| Exact Mass | 273.074951 |
| PSA | 109.57000 |
| LogP | -0.74 |
| Appearance of Characters | yellow |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.691 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ≥14mg/mL |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | NR3397905 |
| HS Code | 2925190090 |
|
~% 
Pomalidomide CAS#:19171-19-8 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 11 p. 1625 - 1630 |
|
~93% 
Pomalidomide CAS#:19171-19-8 |
| Literature: Celgene Corporation an orgnization of the state New Jersey Patent: US2007/4920 A1, 2007 ; Location in patent: Page/Page column 13 ; |
|
~% 
Pomalidomide CAS#:19171-19-8 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 11 p. 1625 - 1630 |
|
~% 
Pomalidomide CAS#:19171-19-8 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 11 p. 1625 - 1630 |
|
~% 
Pomalidomide CAS#:19171-19-8 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 11 p. 1625 - 1630 |
|
~% 
Pomalidomide CAS#:19171-19-8 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 11 p. 1625 - 1630 |
| HS Code | 2925190090 |
|---|---|
| Summary | 2925190090 other imides and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Rational combination treatment with histone deacetylase inhibitors and immunomodulatory drugs in multiple myeloma.
Blood Cancer J. 5 , e312, (2015) Immunomodulatory drugs (IMiDs) thalidomide, lenalidomide (Len) and pomalidomide trigger anti-tumor activities in multiple myeloma (MM) by targetting cereblon and thereby impacting IZF1/3, c-Myc and IR... |
|
|
Pomalidomide: evaluation of cytochrome P450 and transporter-mediated drug-drug interaction potential in vitro and in healthy subjects.
J. Clin. Pharmacol. 55(2) , 168-78, (2015) Pomalidomide offers an alternative for patients with relapsed/refractory multiple myeloma who have exhausted treatment options with lenalidomide and bortezomib. Little is known about pomalidomide's po... |
|
|
A validated UPLC-MS/MS assay using negative ionization mode for high-throughput determination of pomalidomide in rat plasma.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 983-984 , 76-82, (2015) In this study, a sensitive UPLC-MS/MS assay was developed and validated for high-throughput determination of pomalidomide in rat plasma using celecoxib as an internal standard (IS). Liquid liquid extr... |
| 4-Amino-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione |
| 1H-Isoindole-1,3(2H)-dione, 4-amino-2-(2,6-dioxo-3-piperidinyl)- |
| Pomalidomide |
| 1H-Isoindole-1,3(2H)-dione, 4-amino-2-(2,6-dioxo-3-piperidinyl)- |
| Pomalyst |
| 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione |
| 3-Amino-N-(2,6-dioxo-3-piperidyl)phthalimide |
| CC-4047 |